Abstract
Gastroesophageal reflux disease (GERD) is one of the most frequent gastrointestinal disorders. Proton pump inhibitors (PPIs) are effective in healing lesions and improving symptoms in most cases, although up to 40% of GERD patients do not respond adequately to PPI therapy. Refractory GERD (rGERD) is one of the most challenging problems, given its impact on the quality of life and consumption of health care resources. The definition of rGERD is a controversial topic as it has not been unequivocally established. Indeed, some patients unresponsive to PPIs who experience symptoms potentially related to GERD may not have GERD; in this case the definition could be replaced with “reflux-like PPI-refractory symptoms.” Patients with persistent reflux-like symptoms should undergo a diagnostic workup aimed at finding objective evidence of GERD through endoscopic and pH-impedance investigations. The management strategies regarding rGERD, apart from a careful check of patient's compliance with PPIs, a possible change in the timing of their administration and the choice of a PPI with a different metabolic pathway, include other pharmacologic treatments. These include histamine-2 receptor antagonists (H2RAs), alginates, antacids and mucosal protective agents, potassium competitive acid blockers (PCABs), prokinetics, gamma aminobutyric acid-B (GABA-B) receptor agonists and metabotropic glutamate receptor-5 (mGluR5) antagonists, and pain modulators. If there is no benefit from medical therapy, but there is objective evidence of GERD, invasive antireflux options should be evaluated after having carefully explained the risks and benefits to the patient. The most widely performed invasive antireflux option remains laparoscopic antireflux surgery (LARS), even if other, less invasive, interventions have been suggested in the last few decades, including endoscopic transoral incisionless fundoplication (TIF), magnetic sphincter augmentation (LINX) or radiofrequency therapy (Stretta). Due to the different mechanisms underlying rGERD, the most effective strategy can vary, and it should be tailored to each patient. The aim of this paper is to review the different management options available to successfully deal with rGERD.
Keywords: gastroesophageal reflux disease (GERD), refractory GERD (rGERD), 24-h multichannel intraluminal impedance-pH (MII-pH), proton pump inhibitors (PPIs), laparoscopic antireflux surgery (LARS)
Introduction
Gastroesophageal reflux disease (GERD) is one of the most frequent gastrointestinal diseases (1). It is defined on the basis of both esophageal and extra-esophageal symptoms, and/or lesions resulting from the reflux of gastric contents into the esophagus. GERD symptoms can be typical, such as heartburn and regurgitation, and atypical, such as chest pain, chronic cough, laryngeal burn, globus, and hoarseness. Therapy is commonly based on proton pump inhibitors (PPIs) and alginates as an add-on therapy. PPIs are effective in healing lesions and improving symptoms in most cases (2). However, there is a significant proportion of patients, ranging from 10 to 40%, whose symptoms do not adequately respond to PPI therapy (3–6). This condition, commonly known as “refractory GERD” (rGERD), represents a major health problem, given its impact on quality of life and consumption of health care resources (7). The definition of rGERD is controversial as it has never been clearly established (8). The most commonly used definition is: symptoms (retrosternal heartburn and/or regurgitation) present at least 3 times per week not responding to a double dose of PPIs for 8–12 weeks (4, 7–10). It must be emphasized that this definition is only clinical, and it does not take into account the need to have objective evidence of GERD based on endoscopic findings and pH-impedance monitoring. Indeed, many patients who experience symptoms potentially related to GERD and not responding to PPI are not really affected by GERD (7, 9). In this case the definition could be changed to “reflux-like PPI-refractory symptoms.” The latest ESNM/ANMS consensus paper (11), in accordance with recent recommendations (12–14), defined “refractory GERD symptoms” as the persistence of symptoms on therapy, in patients with prior objective evidence of GERD (erosive esophagitis, peptic stricture, long segment Barrett's esophagus, or abnormal esophageal acid exposure on reflux monitoring performed off therapy) and rGERD as “persistence of GERD symptoms with objective evidence of GERD (through endoscopic and pH-impedance findings) despite optimized PPI therapy over at least 8 weeks.” The complex pathogenetic mechanisms underlying rGERD represent a major challenge in gastroenterological clinical practice and need to be further investigated in order to guide effective therapeutic interventions (15). The present paper was aimed at reviewing the treatment of rGERD in light of the most recent research.
Clinical tip: rGERD is referred to the persistence of GERD symptoms with objective evidence of GERD despite optimized PPI therapy over at least 8 weeks.
Diagnostic Workup
Patients with persistent reflux symptoms should undergo a diagnostic pathway aimed at confirming the diagnosis of GERD, evaluating potential comorbidities (including obesity, cardiological and respiratory diseases, psychological/psychiatric disorders) (16), presence of gut-brain axis disorders (17–20) and the use of concomitant medications (21) (Table 1).
Table 1.
Drugs commonly associated with esophageal damage and/or onset of reflux-like symptoms.
| Statins (i.e., Simvastatin, Rosuvastatin) |
| Angiotensin converting enzyme-inhibitors (i.e., Ramipril) |
| Selective serotonin reuptake inhibitors (i.e., Fluoxetine) |
| Calcium channel blockers |
| Antiplatelets (i.e., Clopidogrel) |
| Antibiotics (i.e., Clindamycin or Doxycycline) |
| Ferrous sulfate |
| NSAIDs (i.e., Aspirin, Ibuprofen, Naproxen) |
| Theophylline |
| Nitrates |
| Phosphodiesterase 5 inhibitors (i.e., Sildenafil, Tadalafil) |
| Diphosphonates |
NSAIDs, non-steroidal anti-inflammatory drugs.
An esophagogastroduodenoscopy (EGD), with mucosal biopsies when necessary, should be suggested in order to identify the presence of erosive esophagitis (and its complications) and/or to rule out other causes of esophageal damage, like eosinophilic esophagitis (22, 23). If negative, other tests should be performed (24). High-resolution esophageal manometry (HREM) should be carried out to detect non-reflux esophageal disorders (10, 25), such as esophageal motility disorders, which may have similar complaints of patients with GERD (26–29). If symptoms are suspected of being due to delayed gastric emptying, an assessment of gastric emptying (scintigraphy or breath test) is recommended (30, 31) (Table 2).
Table 2.
Possible causes of persistent GERD-like symptoms and corresponding diagnostic tests.
| Possible causes of persistent GERD-like symptoms | Diagnostic test |
|---|---|
| Eosinophilic esophagitis | EGD |
| Zollinger-Ellison syndrome | |
| Pill-induced esophagitis | |
| Skin disease with esophageal involvement | |
| Infectious esophagitis | |
| Esophageal cancer | |
| Radiation-induced esophagitis | |
| Caustic agent ingestion | |
| Esophageal motility disorders (Achalasia, hypotensive LES, reduced esophageal contractility) | HREM |
| Supragastric belching | |
| Rumination syndrome | |
| Reflux hypersensitivity | MII-pH |
| Functional heartburn | |
| Delayed gastric emptying | Gastric emptying scintigraphy or Breath test |
EGD, esophagogastroduodenoscopy; GERD, gastroesophageal reflux disease; HREM, high resolution esophageal manometry; MII-pH, 24-h multichannel intraluminal impedance-pH; LES, lower esophageal sphincter.
HREM is also mandatory in order to correctly perform a 24-h multichannel intraluminal impedance-pH (MII-pH) monitoring, the current gold standard test to verify the presence of reflux and its association with symptoms (13, 32, 33). MII-pH monitoring assesses the chemical composition of reflux (acidic, weakly acidic, or non-acidic based on pH), its physical composition (liquid, gaseous, or mixed), and its migration to the distal esophagus (34). This technique, in combination with the analysis of the relationship between symptoms and reflux events [symptom index (SI) or symptom association probability (SAP)], determines the association of reflux events with GERD symptoms (7, 15, 35, 36). The GERD Consensus Group considers an acid exposure time (AET) to be normal when <4% and pathological when >6%. For borderline values (between 4 and 6%) the Group suggests considering other metrics, e.g., the number of reflux episodes (acidic, weakly acidic or weakly alkaline), where more than 80 reflux episodes per 24 h are definitely considered abnormal. However, it has also been reported that the total number of reflux episodes alone is not sufficient to confirm the diagnosis of GERD and it should be considered only as an exploratory tool (13, 37).
MII-pH monitoring leads to a stratification of patients into ongoing acid reflux, ongoing symptomatic non-acid reflux (when AET is normal but the patient reports symptoms corresponding to non-acid or weakly acid reflux events) and non-reflux (when AET is normal and reported symptoms are independent of reflux events) (25, 38–41). The test carried out “off treatment,” i.e., after having suspended any acid-suppressive therapy for at least 20 days, confirms the diagnosis of GERD (NERD type), discriminating between GERD functional esophageal disorders like reflux hypersensitivity (RH) and functional heartburn (FH) (42). The latest ESNM/ANMS consensus paper emphasizes the importance of considering additional impedance parameters, such as SAP, post-reflux swallow-induced peristaltic wave index, and mean nocturnal baseline impedance, in order to help identify the patients with rGERD presenting with an inconclusive “off treatment” test (11). MII-pH performed “on – treatment” (during PPI intake) aims to find the causes of a treatment failure (25, 43) and is reserved for patients previously diagnosed with GERD (e.g., affected with Los Angeles Grade C or D esophagitis, peptic stricture, Barrett esophagus, or pathological acid exposure off PPI on esophageal pH monitoring) not responsive to an optimized PPI trial. MII-pH monitoring is essential to assess not only a PPI-refractory acid exposure but also an excessive burden of weakly-acidic contents (37). Identifying the rGERD patient phenotype through MII-pH monitoring is crucial to enable proper management on the basis of the pathophysiological mechanisms (25, 44).
Clinical tip: 24 h MII-pH monitoring “on - treatment” is the gold standard in the diagnostic pathway for rGERD, it being crucial in confirming the presence of reflux and its association with symptoms.
Potential Causes of rGERD
PPI Compliance
In rGERD patients it is necessary to evaluate compliance with the therapy, both in terms of dose and timing of drug intake (45). Dose and timing are key factors in obtaining an adequate response. PPI should be taken when fasting, at least 30 min before a meal, preferably in the morning before breakfast or, in the case of a second dose in the evening before dinner. This is to achieve maximum gastric acid suppression, blocking the proton pumps before food activates them (3). Up to 54% of patients take PPI incorrectly (46) and several studies have shown a poor adherence to PPI therapy, with two reports finding that only 53.8 and 67.7% of patients adhered correctly kept to the prescription for more than 80% of the expected time (47, 48). In addition, many patients stop treatment when their symptoms improve. This was shown by a large population survey in which only 55% of patients took PPI once a day for 4 weeks as prescribed, with 37% taking the drug for 12 days or less (49). Moreover, a study involving 100 patients with persistent GERD-related symptoms found that only 8% of them took the PPI therapy 30–60 min before meals as prescribed (47). Factors related to poor adherence are mainly the presence of mild symptoms, the onset of side effects, poor information, and poor knowledge on the part of the physician regarding the more detailed pharmacological characteristics of PPIs (50).
PPI Metabolism
The pharmacological activity of PPIs differs among patients. A potential contributing factor in this regard is the genotypic variability of cytochrome P450 (CYP) (51). PPIs are metabolized by the liver enzyme complex of CYP, mainly by CYP2C19, and to a lesser extent by CYP3A4. There are three polymorphic variants of CYP2C19 corresponding to 3 different phenotypes: extended or rapid metabolizer (RM) (homozygous, with two mutated alleles), intermediate metabolizer (IM) (heterozygous, one mutated allele and one wildtype) and slow metabolizer (SM) (two wild-type alleles). The rapid metabolizing variant is present in about 60–70% of Caucasians and 30–40% of Asians (52). In a recent meta-analysis, it was found that the response rate to PPIs in RM is lower (52.2%) than in IM (56.7%) and in SM (61.3%) (53). The role of CYP2C19 activity is still unclear. There is only one study in which a discrepancy between a single and double dose of Pantoprazole in RMs is observed, and this could represent a starting point for future studies (54). Up to date genotyping of CYP2C19 is possible by polymeric chain reaction on a peripheral blood sample, but it is not yet widely available for clinical practice (55).
Different PPI Agents
Evidence of the superiority of a PPI agent in comparison with others for rGERD is scarce, but some data suggest that different types of PPI activity can vary widely in terms of acid suppression (56), so some patients could exhibit significant variability in their clinical response to different molecules. Hence, when considering large cohorts of patients there is no evidence of superiority of one PPI over another, whereas in the individual patient it is sometimes useful to switch to a different PPI to obtain better symptom control (57).
Persistent Weakly Acid or Non-acid Reflux
Stimuli other than strong acidity can also be responsible for reflux symptoms, i.e., weakly acid reflux (WAR) or weakly alkaline reflux (WalkR). WalkR has a pH > 7 and WAR has a pH between 4 and 7. It has been observed that between 30 and 40% of patients with rGERD have symptoms related to events of WAR and WalkR (7–10, 15, 58–60). It should not be forgotten that in patients taking PPI, reflux episodes are more frequently of weakly acidic and weakly alkaline nature than acidic as a direct consequence of acid suppression (35). The most widely accepted hypothesis is that distension of the esophagus due to reflux volume and esophageal hypersensitivity to reflux are the conditions that trigger the symptoms (61). It has also been hypothesized that lower pH values of WAR could be a determinant factor in provoking heartburn (39, 62, 63). During a weakly acidic reflux event, pepsin can migrate into the esophagus. This has a proteolytic effect, and although its maximum activity is between pH 1.9–3.6, it displays partial activity even above pH 6 (61). Moreover, it has also been observed that up to pH 6.5, mucosal repair may be impaired (61) and previous exposure of the mucosa to acidic pH may result in the development of hyperalgesia to both mechanical and chemical stimulation (64).
Duodenogastroesophageal or Bile Reflux
Many physicians confuse WAR and bile reflux. The latter is weakly alkaline and can be found especially in patients who have undergone gastrectomy. Bilirubin and bile acids can damage the esophageal mucosa, causing apoptosis, appearance of dilated intercellular spaces (DIS), and increased mucosal permeability (7, 53, 65–67). Data on the role of bile reflux in rGERD are controversial. In a study by de Bortoli et al. bile reflux was shown to play a role in the origin of reflux symptoms not responding to acid suppression (68). However, Gasiorowka et al. found no difference in the degree of duodenogastroesophageal reflux and acid exposure during treatment between patients who failed to respond and those who achieved complete symptom resolution after a PPI trial (69).
Pathophysiology of rGERD
Antireflux Barrier
The weakening of the physiological antireflux barrier seems to play a role in promoting rGERD (3, 9). The antireflux barrier is a high-pressure area consisting of the lower esophageal sphincter (LES) attached to the crural diaphragm through the esophageal ligament, and it prevents gastroesophageal reflux. A reduction in the mucosal barrier's integrity is mainly determined by reduced LES resting pressure, especially in the presence of hiatal hernia, and transient relaxation of LES (TLESR), mainly in the presence of an intact hiatus; this may lead to an increase in volume and exposure time of the refluxed material (3, 12, 21, 70). Zerbib et al. (11) reiterated the potential contribution of obesity (especially central obesity) in promoting rGERD through increasing gastric pressure, leading to higher TLESR events. Further studies are underway to clarify the importance of a weakening of the antireflux barrier in the pathogenesis of rGERD (24, 71–73).
Esophageal Clearance
It has been observed that a delayed clearance of acid and bolus from the esophagus may be associated with a persistence of pathologic reflux, particularly following conditions such as altered esophageal peristalsis, hiatal hernia, and alterations in salivation (74–78). One study has also observed that PPI- refractory patients have lower basal levels of esophageal clearance, which may worsen the effect of reflux (79).
Delayed Gastric Emptying
The association between delayed gastric emptying and rGERD remains unclear (80). It has been hypothesized that delayed gastric emptying, or gastroparesis, may contribute to rGERD through increased gastric distension and consequent TLESR events, which promote reflux of gastric content into the esophagus (81). In one study the use of a prokinetic agent, accelerating gastric emptying prior to pH-impedance, and manometry tests significantly reduced AET and clearance (82).
Esophageal Hypersensitivity and Hypervigilance
There is growing consensus that esophageal hypersensitivity plays an important role in rGERD, particularly in the presence of NAR related symptoms (83, 84). It is defined as the increased perception of different kinds of stimuli, including acid, temperature, mechanical distension, and electrical stimulation (70). The pathophysiologic mechanism seems to involve central and peripheral sensitization through the presence of DIS and exposure of subepithelial nerves to acid. Chronic psychological stress can induce disruption of the intestinal epithelial tight junction proteins, leading to increased intestinal epithelial permeability and associated visceral hyperalgesia (7, 65–67, 84). Recently, the new concept of esophageal hypervigilance, defined as the cognitive-affective process that results from over-awareness of the discomfort, was introduced by Keefer et al. (85) as a possible factor involved in rGERD. The analysis of 70 patients with PPI refractory symptoms studied with pH-impedance and psychometric tests showed that the hypervigilance was shared by all phenotypes, regardless of the patient's phenotype in terms of positive or negative correlation of symptoms and normal or abnormal acid exposure (85).
Therapy of rGERD
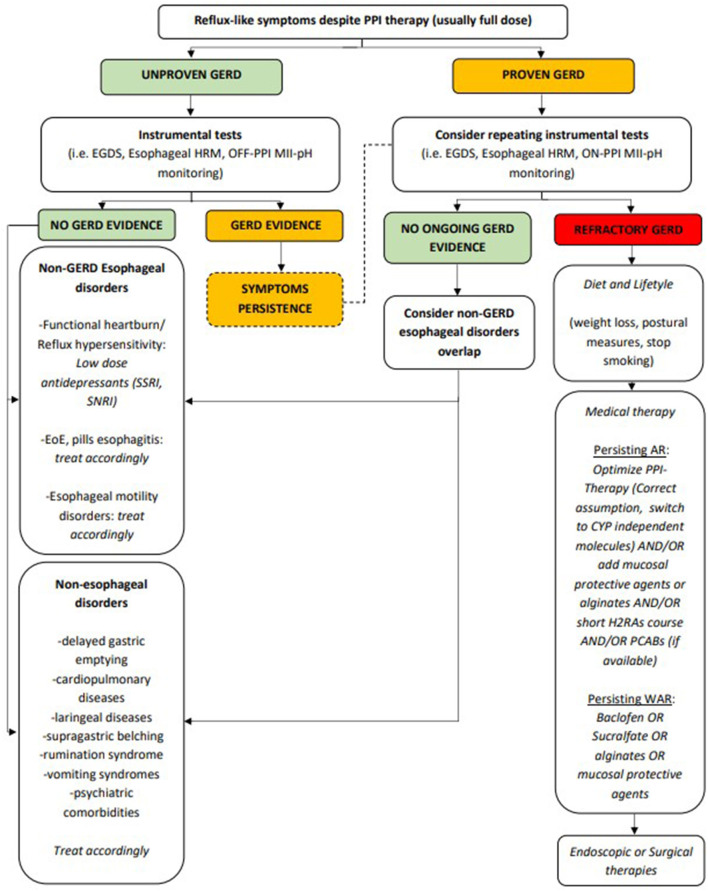
As previously described, several different mechanisms can lead to rGERD and therapy should target specific pathophysiological events. However, in clinical practice, drug use is often aimed at controlling refractory symptoms, especially refractory heartburn or refractory epigastric pain (Figure 1).
Figure 1.
Proposed management of rGERD symptoms. AR, acid reflux; CYP, cytochrome P450; EGDS, esophagogastroduodenoscopy; EoE, eosinophilic esophagitis; GERD, gastroesophageal reflux disease; H2RAs, histamine receptor 2 antagonists; HRM, high resolution manometry; MII-pH, multichannel intraluminal impedance-pH; PCABs, potassium-competitive acid blockers; PPI, proton pump inhibitor; SNRI, serotonin and norepinephrine reuptake inhibitor; SSRI, selective serotonin reuptake inhibitor; WAR, weakly acid reflux.
Diet and Lifestyle
In patients with GERD the first life-style change should consider modification in body weight and elevation of the head of the bed (86–88). Weight loss of at least 10% is recommended in all patients with GERD in order to boost the effect of PPI on symptom relief and to reduce chronic medication use (88). Indeed, potentially effective dietary measures in improving GERD symptoms are those aimed at weight loss, although with a moderate level of evidence (89, 90), and those improving esophageal motility, such as supplementation of dietary fibers. A recent study shows that Psyllium 15 g/day can significantly increase LES minimal resting pressure in NERD patients, leading to a reduction in the number of acid and weakly acid reflux events and in the frequency of pyrosis (91). Recently, a study based on MII-pH post-prandial analysis by Martinucci et al. showed that a meal based on vegetable proteins was associated with a lower number of refluxes, particularly acid refluxes, and with a reduced number of symptoms during the first postprandial hour, compared to one with a prevailing component of animal proteins (92). Also, regarding avoidance of alcohol, peppermint, coffee or fatty food, evidence is scarce and this suggestion, despite being very frequently proposed in clinical practice, cannot be strictly recommended (90, 93, 94). A clinical trial showed that fermentable oligo-, di-, mono- saccharides and polyols (FODMAPs), particularly fructans, increased the number of TLESRs in healthy patients (95), and in proven rGERD patients a low- FODMAP diet was shown not to significantly decrease reflux symptoms when compared to usual dietary advice (96). In conclusion, no clear scientific evidence regarding a positive role of dietary suggestions in rGERD exist to date, even if also in these patients they are frequently recommended because they are low cost and can lead to positive effects on individual health. On the contrary, the association between tobacco smoking and GERD is well-documented by a recent meta-analysis (97). Reinforcing avoidance of tobacco consumption in refractory patients could thus be a cost/effective measure.
Clinical tip: weight loss, postural measures and smoking cessation are cost-effective also in rGERD patients.
PPI Therapy
As previously described, PPIs are the standard therapy for GERD. When symptoms persist, revealing a condition of rGERD, many different possible therapeutic options are available despite their efficacy often being weak (98).
Reinforcing Correct Drug Intake
Low adherence regarding PPI intake is described in up to 47.5% of cases, as mentioned above (99). Stressing the instructions for correct drug intake (i.e., taking it with an empty stomach, followed by a meal within 30 min) is however essential (54), in order to obtain maximum cost-effectiveness in acid gastric suppression.
Doubling PPI-Dose and High PPI-Doses
According to the above-mentioned definition, patients with rGERD should already have experienced a double-dose PPI treatment. A twice-daily PPI dose is not recommended by European and North American Drug Authorities. It can however lead to adequate symptom control after 6–8 weeks in 20–30% of patients who have been on a standard daily dose, when persistent acid exposure is proved by MII-pH monitoring (100). Whether clinical remission is achieved or not, titration should be proposed after 2–3 months, in order to avoid possible long-term PPI side effects (25, 101). Finally, only a recent controlled trial recorded a strong pH-lowering (MII-pH monitoring) when Esomeprazole 20 mg q.i.d. was administered to Helicobacter Pylori (HP) negative patients (102). The authors concluded that this might be one of the rescue regimens for patients who are refractory to PPI treatment, irrespective of CYP2C19 genotype. However, caution is mandatory until more robust evidence is available.
Changing PPI Formulation
In order to overcome compliance problems, new drug formulations such as Dexlansoprazole modified-release (MR) have recently been developed. This MR formulation allows for a once-daily dosage regardless of time of day or food consumption. However, the drug has been mainly tested in patients with erosive esophagitis or nocturnal GERD symptoms, rather than in patients with true rGERD (103). It is also more expensive than standard PPI formulations and it is not widely available in all European markets. No other PPI MR formulations have been studied to date.
Splitting PPI-Dose
This can be suggested when there are persistent nocturnal symptoms, possibly associated with NAB. Papers describing the effects of splitting PPI doses on gastric acidity and especially on NAB, differ according to the different PPI molecules. A pH increase has been described when Omeprazole 40 mg once a day is split into two doses (i.e., before breakfast and dinner) (104), but not when splitting Lansoprazole 30 mg or Pantoprazole 40 mg (105). This is despite the same efficacy as Omeprazole being achieved when these molecules are given once a day. A more recent study in healthy volunteers showed that Esomeprazole can lead to a better acid secretion control in the initial 7-day period of the treatment when the daily dose (40 mg) is split into a morning and an evening dose (106).
Changing PPI-Molecule or PPI-Brand
Although evidence is quite limited for a PPI agent being superior to the others for rGERD, some data suggest that relative potencies of different PPIs vary widely (107, 108). Therefore, as previously mentioned, patients can exhibit significant variability in their clinical response to different molecules (56). However, a recent meta-analysis found no differences in the effectiveness of acid suppression when comparing equivalent doses of different types of PPIs, indicating that these can be used interchangeably (56). Moreover, the 2005 Canadian Consensus Conference (109) and the statements of the World Health Organization Collaborating Centre for Drug Statistics Methodology have suggested that PPIs are more similar than different (110). Pending more robust data, an attempt to change the molecule can be made, although it is difficult to quantify the effectiveness of this measure. In these cases, drug doses should be titrated according to the described relative power. Choice of a CYP-independent PPI requires further consideration and can be seen as a different strategy, as described in the next paragraph. Finally, there are very few reports regarding the possible efficacy of simply changing the PPI brand within the same molecule (111). In our opinion, in this case, when patients report improvement of symptoms, a placebo effect and/or psychological component could play an important role, especially when there are other functional disorders and/or psychiatric comorbidities involved.
Switch to a CYP-Independent PPI
Most PPIs, except Esomeprazole and Rabeprazole and where available Ilaprazole and Azelaprazole, undergo hepatic metabolism through the CYP enzymatic complex, mainly through CYP2C19 (112) and CYP3A4 isoenzymes (113). As previously described, patients can be classified into RM, IM and SM. There is a higher prevalence of RM patients among Caucasians (60–70% of the general population) and Asian populations (82). Genetic testing evaluating the CYP2C19 genotype is quite expensive and seldom available thus making a PPI shift to CYP independent molecules the commonest clinical approach (55). Taken together, both doubling PPI dose (among common CYP- dependent molecules) and switching to a CYP-independent molecule account for a 25% improvement overall in satisfactory clinical response and, despite the greatest potential gain for the second strategy, there is no solid evidence to date for either one or the other (100). The most important aspect to consider is that the relative response of different symptoms to acid suppression correlates with their association with acid reflux (114, 115) and up to 80% of patients with persistent symptoms, despite optimized PPI therapy, probably have non-acid reflux. In these cases other therapeutic approaches should be considered.
Clinical tip: PPI are more effective when taken with an empty stomach and 30 min before breakfast. It could also be useful switching to CYP independent PPI or splitting PPI into two doses if nocturnal symptoms are present.
Histamine-2 Receptor Antagonists
An eventual effective therapeutic strategy can involve the addition of a Histamine-2 receptor antagonist (H2RA) at bedtime to control nocturnal symptoms (14). This is because histamine is an important driver of nocturnal acid secretion and may explain the scarce responses to PPI bedtime administration (99). H2RAs could also modulate esophageal sensitivity to acid in patients with GERD or FH (116). Some studies have shown that the addition of a nighttime H2RA dose to twice- daily PPI decreases nocturnal acid breakthrough (NAB) from 64 to 17% (55, 117–119). However, other research has not clearly detected a correlation between NAB and symptoms and has not shown a significant reduction in AET and SI (117). Moreover, tachyphylaxis phenomena can develop in <10 days after starting H2RA therapy (118), leading to drug discontinuation in up to 13% of patients (119). Hence, H2RA would be better taken on demand or intermittently (9), regardless of whether it is an add-on therapy to a double PPI therapy or a substitute for the second PPI dose (25). It is worth noting that administration of H2RAs together with PPIs is considered to be safe (120).
Clinical tip: addition of a H2RAs at bedtime can be considered to better control nocturnal symptoms.
Alginates, Antacids, and Mucosal Protective Agents
This is a wide class of drugs with different mechanisms of action, effective in controlling residual symptoms generally without serious side effects. Solutions containing sodium alginate precipitate into a viscous gel that may create a physical barrier to the so-called “acid pocket,” which may not be completely eliminated by PPIs (79). These drugs decrease the severity and frequency of heartburn when used postprandially as add-on therapy to PPIs in patients with GERD (121). It is noteworthy to mention that alginates available on the U.S. and European markets are different, with the latter containing higher alginate concentrations and leading possibly to a higher efficacy (35). Despite some studies reporting prompt efficacy in controlling both postprandial and nocturnal symptoms in patients with rGERD (121), none of these objectively assessed presence and type of reflux with MII- pH monitoring. Given their lack of significant adverse events, they can however be used as an add-on to PPIs according to local availability and the physician's and patient's preference. Sucralfate is a mucosal protective agent that acts by blocking the diffusion of gastric acid and pepsin through the esophageal mucosa and stimulates mucosal growth factors, thus promoting the formation of mucus and bicarbonate (122). It is a safe drug, with a good efficacy in controlling GERD symptoms and a potential effectiveness in improving mucosal healing of erosive mild grade esophagitis. Despite the lack of data regarding its use in patients with rGERD (123), sucralfate may be a safe option for symptom management in pregnant women (124) and, as a complementary therapy in patients with drug-induced esophagitis (122). Due to the ability of binding bile salts, it could be of help also in WAR and/or WalkR. A new class of disposable medical agents, made up of a bioadhesive formulation of hyaluronic acid and chondroitin sulfate, was recently shown to improve symptoms and quality of life as an add-on therapy to PPI when compared to PPI alone in patients with NERD (125, 126). Despite their use in patients with rGERD not having been evaluated yet, they could be of help in some patients because they target an additional pathophysiological mechanism.
Clinical tip: adding alginates, antacids or mucosal protective agents can be effective in controlling residual symptoms, generally without serious side effects.
Potassium-Competitive Acid Blockers
Potassium-competitive acid blockers (PCABs) competitively inhibit proton pumps (hydrogen- potassium ATPases) and have been approved in Japan since 2015 for the treatment of peptic ulcer and reflux esophagitis and eradication of HP infection (127, 128). Given their clinical efficacy, experts suggest that they could also be more useful than PPIs as an initial empirical test before performing instrumental diagnostic tests to exclude acid-mediated reflux disease (129). Vonoprazan, which is not yet available on the European market, has shown a greater ability in suppressing acid secretion through blocking both inactive and active proton pumps, with a greater duration of action compared to PPIs (130–132). Its elimination is independent of CYP2C19 and this may contribute to explaining its stronger effect (133). Some retrospective studies have demonstrated symptomatic improvement in patients with rGERD (134) and efficacy in the treatment of persistent symptomatic erosive esophagitis when drug intake is continued for at least 8 weeks. Furthermore, a very recent small (30 patients) uncontrolled study showed the long-term efficacy with 1 year of Vonoprazan in rGERD (135). However, a study in rGERD patients complaining of dyspepsia was not able to detect any significant effect of Vonoprazan (136) and the drug failed to normalize AET in patients with absent esophageal contractility (i.e., patients with systemic scleroderma) (137).
Clinical tip: whenever available, vonoprazan is more effective than PPIs in acid suppression.
Prokinetics
Prokinetic agents, approved for the treatment of patients with gastroparesis (138), have also been suggested as an add-on therapy in some patients with rGERD, since delayed gastric emptying can lead to symptom persistence. These drugs could act not only by improving gastric emptying, but also by increasing LES pressure and improving esophageal clearance. According to the different molecules, they can act by binding to different receptors, including 5-hydroxytryptamine (5-HT) 4, dopamine 2 (D2), motilin and ghrelin receptors (80). However, a recent meta-analysis found that the addition of a prokinetic to a PPI does not provide clear benefits in symptom control, but it improves quality of life (139). Hence, in rGERD patients with normal gastric emptying, adding these agents for a short time-period deserves to be carefully evaluated in terms of risk-benefit balance.
Anti Dopamine 2 Receptor Agents
Metoclopramide, an anti-dopaminergic drug, has been studied in association with H2RAs, but it did not significantly improve symptoms over H2RAs alone (140). Studies to assess its efficacy as an add- on therapy to PPIs in rGERD patients are lacking. However, its long-term use (i.e., more than 12 weeks) could be limited by the onset of side effects like insomnia, agitation and tardive dyskinesia. Also, Domperidone has been shown to be effective in increasing LES pressure (141), but a recent study indicated that it did not improve reflux symptoms when added to PPI in rGERD (142), thus limiting its use within this context.
5-HT Receptor Active Drugs
Cisapride, a non-selective 5-HT4 agonist, was the first effective prokinetic to be used as an add-on therapy in patients complaining of nocturnal heartburn (143). However, due to the report of a few cases of QT prolongation and fatal arrhythmias, it was withdrawn in the early 2000s. Other non- selective 5-HT4 agonists have also been evaluated. Mosapride also acts on 5-HT3 receptors through its metabolites, stimulating esophageal clearance and gastric emptying rate. Despite initial doubtful results (118), it seems to be effective in controlling rGERD symptoms when used as an add-on therapy (144), but its efficacy in true refractory patients still has to be proved. On the contrary, Revexepride, another 5-HT4 agonist, failed to achieve clinical benefits in r-GERD (145). Finally, Tegaserod, acting on both 5-HT4 and 5-HT2B receptors, has been shown to be effective in decreasing reflux events and TLESR, but it has never been tested within the setting of rGERD. In more recent times, Prucalopride, a highly selective 5-HT4 agonist approved for the treatment of constipation, was also shown to be effective in reducing AET and in stimulating gastric emptying when used at high doses (e.g., 4 mg/die) in healthy volunteers (82). Despite its potential in stimulating secondary peristalsis in GERD patients, further studies are needed to assess its efficacy in achieving symptom control, particularly with regard to rGERD.
Clinical tip: prokinetics may be used if delayed gastric emptying is associated.
Gamma Aminobutyric Acid-B Receptor Agonists and Metabotropic Glutamate Receptor-5 Antagonists
Gamma aminobutyric acid-B (GABA-B) receptor agonists and metabotropic glutamate receptor-5 (mGluR5) antagonists are drugs able to reduce the number of reflux events, irrespective of whether they are acid, weakly acid or alkaline. However, patients who could benefit the most are those with symptomatic weakly acid or bile reflux. Baclofen, a GABA-B receptor agonist, has been shown to decrease acid reflux events, esophageal acid exposure and reflux-related symptoms, both as a monotherapy for 2–4 weeks or as an add-on therapy to PPIs in patients with persistent symptoms (146). Its potential value as an add-on therapy was also confirmed in a recent meta-analysis (147). In order to overcome the short half-life and poor tolerability profile of Baclofen in terms of the central nervous system and abdominal side effects (i.e., dizziness, accommodation disorders, drowsiness, nausea, vomiting or diarrhea), new compounds with a greater binding ability for peripheral receptors have been developed. Among these, Lesogaberan, a GABA-B receptor agonist, was effective when used as an add-on therapy, with a dose-dependent mechanism (144). A phase IIb trial with more than 500 rGERD patients, showed clinical response only at higher doses (i.e., 240 mg/die), leading to a stop in further developments of Lesogaberan (148). Arbaclofen placarbil is the active R-isomer of Baclofen. Despite being initially found to be effective in controlling reflux events, a trial evaluating its efficacy when used in monotherapy in patients with symptomatic GERD found a lack of effects, decreasing enthusiasm regarding its development (149). Although a post-hoc analysis in patients with rGERD treated with Arbaclofen and PPI showed efficacy in controlling heartburn when compared to placebo (149), its development has also been interrupted. mGluR5 antagonists are drugs developed to act by targeting LES barrier function through inhibiting TLESR. Among these, Mavoglurant (150) and seemed to be promising in controlling GERD. In summary, despite several efforts in the development of new anti-reflux agents, their relatively scarce efficacy or the onset of side effects has led to a high rate of discontinuation (118). Thus, to date, in this class of drugs the only effective option for rGERD patients is that of adding Baclofen 5–10 mg three times a day, with close monitoring of neurological and/or abdominal side effects.
Clinical tip: Among GABA-B receptor agonists baclofen is the only effective option for rGERD patients.
Pain Modulators: Antidepressants and Transient Receptor Potential Vanilloid Receptor-1 Antagonists
Persistence of reflux symptoms may be due also to esophageal hypersensitivity and hypervigilance for which it is hypothesized that mechanisms of central and peripheral sensitization are involved, also in correlation with response to stress. In these cases visceral analgesics [e.g., tricyclic antidepressants (TCAs), selective serotonin reuptake inhibitors (SSRIs), serotonin norepinephrine reuptake inhibitors (SNRIs) or trazodone] can be of help, particularly in avoiding the unnecessary use of high doses of acid inhibitory therapies in patients diagnosed as refractory to PPIs (151). These drugs can exert an analgesic effect through both peripheral and central nervous system pain modulation. To date, evidence for rGERD patients is however only indirect and comes from some placebo-controlled studies. For example, Citalopram 20 mg/day was found to be effective in patients with RH and Venlafaxine 75 mg/day obtained a good control of FH (152). Also Fluoxetine and Sertraline, compared with placebo and PPIs, improved symptoms in patients without pathological AET, in 6–8 weeks (149). It is to be highlighted that the administered doses did not interfere with mental functions because they were lower than those usually administered to obtain anxiolytic and/or antidepressive effects. Currently, there are no indications as to how long this therapy should last. Despite efficacy in reducing esophageal pain perception in healthy volunteers, TCAs (e.g., Imipramine, Nortriptyline) were not particularly able to treat functional esophageal symptoms (153). This is probably due to their action in delaying oro-cecal transit time (154). Transient receptor potential vanilloid receptor-1 (TRPV1) is an acid, heat and capsaicin sensitive receptor involved in the transduction of nociceptive reflux-induced stimuli. AZD1386 is aTRPV1 antagonist which seemed to be effective in increasing pain thresholds in healthy volunteers but did not lead to a significant symptomatic response in one study on rGERD patients (155). To date, no further studies evaluating this drug have been published. Summarizing, low doses of SSRIs and SNRIs are the only agents available and safe for patients complaining of reflux symptoms without evidence of pathological AET. An empirical approach suggested by Scarpellini et al. might be the association of an SSRI (Citalopram or Fluoxetine) at standard morning PPI doses, with follow-up evaluation of symptom improvement after 6 weeks (35). Additional studies are clearly needed, specifically those with a larger number of patients and a better stratification according to reflux parameters and psychiatric disorders.
Clinical tip: if there is esophageal hypersensitivity and hypervigilance, SSRI and SRNI could be used at low doses.
Gastric-Retained Bile Acid Sequestrants
Gastric-retained bile acid sequestrants are an old-new class of drugs binding bile acids in the stomach. They represent an extended-release formulation of the bile acid sequestrant Colesevelam. Preliminary studies have shown potential benefits in rGERD patients. A recent clinical trial of a new compound (IW-3718) also proved to be effective in symptom control when the drug was used as an add-on to a standard PPI dose (98). The short-term use of these agents, when they are available on the market, could thus be a reasonable approach in patients with rGERD (156).
Alternative and Complementary Therapies: Psychological Factors
Alternative therapies including psychotherapies, are becoming increasingly popular among patients with scarce response to PPIs doses or with reflux-like symptoms unrelated to pathological AET (157). Psychological factors can make esophageal symptoms worse and psychiatric comorbidities are often associated with persistent symptoms (158) and poor PPI response. In addition to the use of antidepressants (e.g., SSRI) at full dose (159), many other possible approaches have been suggested in recent years, despite the limited evidence associated with such methods. Among these, cognitive- behavioral therapy involving diaphragmatic breathing (160) and esophageal-directed hypnotherapy (161) could be interesting approaches in selected patients, targeting specific pathophysiological mechanisms such as supra-gastric belching (160) and functional pain, respectively. Hypnotherapy may also play a role in influencing gastric acid secretion and gastric emptying time, probably by modulating esophageal hypervigilance. Biofeedback therapy, a technique which employs visual and audio signals or direct verbal feedback to gain a greater awareness of bodily functions, could be interesting (162). Finally, also acupuncture is reported to improve GERD symptoms, although the exact mechanisms are not known (163). However, studies evaluating the clinical efficacy of these approaches in patients with rGERD are scarce.
Endoscopic and Surgical Management
In the case of failure of medical therapies, invasive antireflux options should be considered (87, 93, 164). The most widely performed invasive antireflux option remains laparoscopic antireflux surgery (LARS) (14, 164–166), even if other, less invasive, methods have been suggested in the last few decades (167, 168). These include endoscopic transoral incisionless fundoplication (TIF), magnetic sphincter augmentation (LINX) or radiofrequency therapy (Stretta). It is mandatory to choose the most suitable option following a discussion with the patient regarding the relative risks and benefits (e.g., long-term efficacy, invasiveness, etc.).
Laparoscopic Antireflux Surgery
The targets of surgical fundoplication are to reposition the LES in the abdomen, closing the hiatal opening, and creating a 1-way flap valve (169). LARS has a success rate ranging from 67 to 95%, greatly depending on adequate patient selection and preoperative evaluation, and on the practitioner's expertise (170–172). Post fundoplication symptomatic recurrence can be caused by an incorrect indication and/or an incomplete preoperative evaluation, or by an inadequate surgical technique (173). Older age, female sex, and presence of comorbidities are reported as risk factors for symptom recurrence (174). In a recent randomized controlled trial (RCT), including patients with GERD refractory to a course of Omeprazole 20 mg twice daily for 2 weeks, the success of the treatment with laparoscopic Nissen fundoplication at 1 year was significantly superior to that obtained with active medical treatment (Omeprazole plus Baclofen, with Desipramine added depending on symptoms), or control medical treatment (Omeprazole plus placebo) (44). LARS can be total or partial. Nissen is a total (360°) fundoplication after crural closure. The two most common partial LARS include a 270° posterior fundoplication (Toupet) and a 180° anterior fundoplication (Dor) (175). A RCT showed a similar effect of partial and total LARS for controlling GERD 3 years after surgery (176). Up to 30% of patients will develop structural complications, often related to surgical positioning or construction of the wrap (177). The EGJ is a complex anatomical area subjected to a multitude of mechanical stresses, related to the gastroesophageal pressure gradient and its possibility of moving axially. Thus, the fundoplication may weaken over time, resulting in wrap disruption, herniation or slippage (178–180). Approximately 5–10% of patients undergoing LARS can receive a second procedure to control their symptoms over time (181). Furthermore, an excessively tight fundoplication could provoke dysphagia (3, 139, 182). According to Håkanson et al., partial LARS is associated with lower rates of post-procedure dysphagia at 2 years in comparison with total LARS (176), particularly regarding anterior fundoplication (183). The partial LARS may be preferred to reduce post fundoplication dysphagia within the setting of an impaired esophageal peristaltic reserve at baseline (175). HREM, performed at the time of diagnosis to rule out esophageal motility disorders is also useful before surgery to assess esophageal contractile vigor and reserve, addressing the choice of the most suitable type of operation (87). Indeed, gastrointestinal symptoms following LARS are not uncommon, including dysphagia, gas-bloating syndrome, chest pain, and diarrhea (177). Consequently, LARS should be recommended with caution, as it can provoke severe adverse effects and the intended effect may be only temporary, because up to 60% of patients will use antireflux medical therapy in the following decade (184). Moreover, among patients who underwent primary LARS, 17.7% experienced recurrent GERD-like symptoms, which required long-term medication or secondary antireflux surgery (174).
Alternative Invasive Procedures
Several attempts have been made to develop minimally invasive, mainly endoscopic, procedures to improve the antireflux barrier (185). In the last two decades, several antireflux approaches have become available. All these procedures act on different mechanisms involved in GERD pathophysiology, in particular EGJ reconstructive therapies and/or LES augmenting therapies.
Endoscopic Transoral Incisionless Fundoplication
TIF is an endoscopic procedure which aims to repair hiatal hernia and restore the LES physical barrier by creating a mechanical valve through a partial fundoplication (186, 187). TIF 2.0, a current technique iteration, is anatomically and physiologically similar to LARS. During the procedure, the gastric fundus is folded up and around the distal esophagus and anchored with polypropylene fasteners. TIF 2.0 has been shown to be a safe and effective treatment only for GERD patients with a hiatal hernia of <2 cm and a Hill grade of <3. In fact, patients with a hernia larger than 2 cm or Hill grade 3–4 should be candidates for LARS or laparoscopic hernia repair with concomitant TIF (188). In a cohort study of 49 patients, followed up to 10 years, most patients (92%) had stopped or reduced the use of PPI therapy after TIF (189). A recent meta-analysis was conducted using data only from RCTs that assessed the TIF 2.0 procedure compared with sham or PPI therapy. Its aim was to determine the efficacy and long-term outcomes associated with TIF 2.0 in patients with rGERD using optimized PPI therapy. Results from this meta-analysis demonstrated that the TIF 2.0 procedure at 3 years produced significant changes in esophageal pH, decreased PPI utilization, and improved quality of life (190). It is to be highlighted that among the studies included in the meta- analysis only one applies the definition of rGERD (187, 191, 192). Furthermore, in another meta- analysis, TIF was not superior to LARS regarding improvement of esophagitis and increase in LES pressure (186, 193). The serious adverse event rate, including gastrointestinal perforation and bleeding, ranges from 2 to 2.5% (194, 195).
Medigus Ultrasonic Surgical Endostapler Procedure
MUSE is a novel endoscopic device that closely mimics surgical anterior fundoplication through transoral stapling. It appears to be safe and technically easy (196). In a 6-month prospective trial, MUSE was reported to have improved symptom control and reduced PPI use in patients with ≥2 years of documented GERD symptoms and ≥6 months of continuous PPI therapy (197). These results were confirmed in a long-term trial involving 37 patients treated with MUSE; they were similar or better in efficacy and safety than TIF 2.0 and Stretta procedures (198). However, larger studies with control groups are needed to determine the real long-term efficacy of MUSE for GERD and rGERD patients.
Endoscopic Mucosal Resection Techniques
Endoscopic mucosal resection therapies differ in technical aspects, but substantially consist in an endoscopic ablation of cardial mucosa, causing LES tightening due to the scarring process, and includes anti-reflux mucosectomy (ARMS) (199), resection and plication (RAP) (200), anti-reflux mucosal ablation (ARMA) (201), antireflux ablation therapy (ARAT) (202), mucosal ablation and suturing of the EGJ (MASE) (203). According to the various authors, these procedures have been performed in PPI-refractory GERD patients, but the current definition of rGERD has only been used in ARMA and ARAT studies. Stenosis and/or dysphagia during post-procedure follow-up have been reported in some patients. The post-procedural efficacy has been variably assessed considering a decrease in AET and/or DeMeester (DM) score, GERD-Health Related Quality of Life Questionnaire, GerdQ and Frequency Scale for the Symptoms of GERD, and PPI discontinuation. Even if most studies report significant improvement in the evaluated parameters, they are flawed by many limitations such as lack of multicenter RCTs, small patient samples and short-term evaluations (199–203).
Endoscopic Full-Thickness Plication (GERDx)
Endoscopic full-thickness plication with the GERDx device was performed in 40 patients with GERD not responding to PPI use for ≥6 months. The most common adverse events were sore throat (20%) and chest pain (17.5%). Ten percentage of patients developed serious postoperative adverse events and about 17.5% underwent LARS before the 3-month follow-up. In the thirty patients available at the 3-month follow-up, an improvement of DM score, reflux related symptoms and gastrointestinal quality of life index were observed. However, three patients needed PPI treatment daily and eight on demand at the 3-month follow-up (204). In conclusion, the GERDx device does not seem to display satisfactory efficacy and safety for rGERD patients.
Magnetic Sphincter Augmentation (LINX)
Magnetic Sphincter Augmentation with the LINX device is a reversible procedure approved by the FDA for treatment of rGERD that does not alter the esophageal and gastric anatomy (177, 205). The device, laparoscopically placed around the LES, is aimed at creating an effective antireflux barrier, thus allowing bolus transit into the stomach and enabling belching and vomiting (206). Recent research by Rogers et al. showed that LINX is indicated in patients with a high number of reflux episodes (specifically > 80) detected on MII-pH monitoring, and reducing the number of reflux events to physiological levels provides an effective improvement in symptom outcome and treatment satisfaction (207). However, the most common side effect of LINX is dysphagia, whereas the most feared complications are device migration and esophageal erosion (~0.15%) (208). A systematic review comparing LINX to LARS reported a similar efficacy in controlling GERD symptoms and esophageal pH, with a larger reduction in gas bloating and improvement in belching in patients receiving the LINX procedure (209). Currently, the device is not licensed for use in severe erosive esophageal disease or large hiatal hernias (210). LINX therefore represents an appealing alternative to LARS, but a RCT between these two interventions for the treatment of GERD is necessary in order to directly evaluate the real efficacy of LINX (209).
Radiofrequency Therapy (Stretta)
Stretta procedure delivers endoscopic radiofrequency energy to the mucosa around the LES, improving the barrier function of the EGJ through scar tissue formation (211). In 2013 the Society of American Gastrointestinal and Endoscopic Surgeons recommended Stretta with a high level of evidence for rGERD (212). However, conflicting results regarding the efficacy of Stretta for the management of GERD symptoms, decrease in PPI use and reduction of AET were all reported (213–215). A revision of 140 Stretta-related papers performed by Das et al. suggest that further studies are required to evaluate the long-term efficacy of Stretta compared to the current best medical and surgical treatments for patients with GERD (216).
Esophageal Neurostimulation
An electrical neuromodulator (EndoStim), implanted into the abdominal wall by laparoscopy with a pair of electrodes placed on the LES, has been approved in Europe and South America. In an uncontrolled before-after study the device has proved to reduce symptom scores and AET in GERD patients with incomplete response to daily use of PPI for ≥12 months. The serious adverse events reported related to a device or procedure were bowel perforation and lead to erosion only in 2 out of 43 patients (217). Overall, the efficacy of neurostimulation in rGERD is still uncertain (218).
Clinical tip: if there is failure of medical therapies, LARS remains the gold standard among invasive antireflux options.
Discussion and Conclusions
rGERD is a complex condition, and undoubtedly its non-univocal definition makes it difficult to compare different studies and draw appropriate and definite conclusions. Solving this not exclusively semantic problem could therefore be an effective starting point for planning consistent and effective clinical trials. In rGERD the persistence of symptoms is often associated with pathophysiological mechanisms involving also non-gastrointestinal systems and is still not fully understood. Moreover, symptoms of rGERD do not depend only on the presence of actual reflux but on other factors that are often difficult to distinguish, i.e., RH and FH. The first line strategy should always include a thorough reassessment of dietary and lifestyle factors, reiterating correct PPI timing and dose modalities. Additional pharmacological therapy should target the potential underlying pathophysiology according to the results of instrumental diagnostic tests (e.g., EGD, HREM, MII-pH monitoring and gastric emptying tests). When considering further therapy for rGERD, however, physicians should keep well in mind that evidence is often weak or indirect, coming from small studies that are often not RCTs. PPI therapy can be widely modulated according to the results of MII-pH monitoring, with a double dose (if not already suggested) if there is ineffective acid suppression or a split dose when there is NAB. Shifting to another PPI molecule, especially a cytochrome-independent molecule, can be suggested especially in populations where the prevalence of RMs is higher. A rescue trial with high-dose PPIs (i.e., q.i.d.) is a less valid option due to its weak evidence of efficacy, the need for high-grade compliance, the higher costs and the possible higher risk of side effects. PPI MR, where available, can be a valid alternative in patients with low compliance, as they require only a single administration per day. It is worth recalling that PPI therapy should always be titrated to the lowest minimal effective dose after a 4–8 week full-dose period, in order to prevent potential long-term side effects. However, their efficacy is not usually long lasting due to tachyphylaxis phenomena. They can thus be used for up to 4-week periods, possibly restarting them after a 4 week washout. Alginates and mucosal protective agents can be a valid alternative when used as an add-on therapy to PPIs within this clinical context, given the absence of significant side effects. Sucralfate, alginates, or mucosal protective agents, together with GABA-B receptor agonists, can also be an effective option if there is persistence of non-acid or weakly acid reflux. Baclofen is the only effective GABA-B receptor agonist available on the market, but due to its many potential side effects, it should be used in very carefully selected cases. Adding a prokinetic with close monitoring for side effects could be an option when there is concomitant delayed gastric emptying or gastroparesis. However, it should be mentioned that these agents are effective only with reflux symptoms associated with altered motor gastric function and their efficacy has not been thoroughly evaluated.
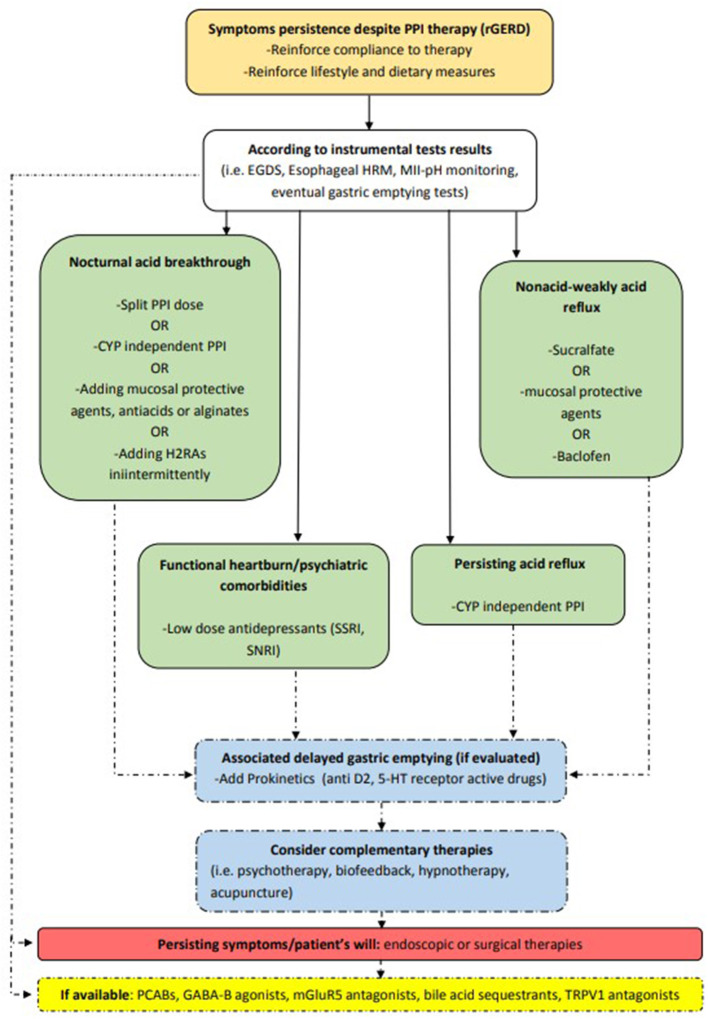
If RH or FH are detected, or the patient suffers from psychiatric comorbidities, pain modulation with antidepressant drugs should be the choice to control symptoms, either singly or in combination with standard PPI doses. Low dose SSRIs seem to be safer than the older tricyclic antidepressants, although a thorough evaluation in rGERD patients is still lacking. Finally, many new agents targeting potential pathogenic mechanisms are currently being developed or are awaiting approval in Western countries and are soon expected to deal with the complexity of rGERD. Among these, PCABs seem to be the most promising agents, but further studies are needed to evaluate their potential long-term side effects. Complementary therapies have not been thoroughly evaluated to date, but some of them (e.g., cognitive-behavioral therapy and biofeedback) are interesting in patients where psychological factors can play an important role in eliciting or worsening symptoms. When medical management fails, invasive antireflux options should be evaluated after having carefully explained their risks and benefits to the patient. An objective confirmation of rGERD is mandatory because surgical and endoscopic antireflux interventions are invasive procedures and outcomes largely depend on appropriate patient selection. The gold standard option remains LARS. This remains true, even if other less invasive interventions, which have not yet demonstrated long-term safety and efficacy, have been suggested in recent years. Figure 2 provides an overview of possible treatments for rGERD based on its pathophysiology.
Figure 2.
Different therapeutic options in rGERD management according to pathophysiology. CYP, cytochrome P450; D2, dopamine 2; EGDS, esophagogastroduodenoscopy; EoE, eosinophilic esophagitis; GABA-B, gamma aminobutyric acid-B; H2RAs, histamine receptor 2 antagonists; HRM, high resolution manometry; mGluR5, metabotropic glutamate receptor-5; MII-pH, multichannel intraluminal impedance-pH; PCABs, potassium-competitive acid blockers; PPI, proton pump inhibitor; rGERD, refractory gastroesophageal reflux disease; SNRI, serotonin and norepinephrine reuptake inhibitor; SSRI, selective serotonin reuptake inhibitor; TRPV1, transient receptor potential vanilloid receptor-1; 5-HT, 5-hydroxytryptamine.
Author Contributions
FR, FB, NB, and MB: conceptualization and methodology. FR, FB, MC, and MB: writing—original draft preparation. CL, AP, GB, ES, NB, and FZ: review and editing of final manuscript. SM: supervision. All authors have read and agreed to the published version of the manuscript.
Conflict of Interest
FZ was employed by company CHU de Bordeaux. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors are grateful to Chris Powell for the language revision.
References
- 1.El-Serag HB, Sweet S, Winchester CC, Dent J. Update on the epidemiology of gastro-oesophageal reflux disease: a systematic review. Gut. (2014) 63:871–80. 10.1136/gutjnl-2012-304269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vakil N, van Zanten SV, Kahrilas P, Dent J, Jones R. Global Consensus Group. The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol. (2006) 101:1900–20. 10.1111/j.1572-0241.2006.00630.x [DOI] [PubMed] [Google Scholar]
- 3.Kahrilas PJ, Boeckxstaens G, Smout AJ. Management of the patient with incomplete response to PPI therapy. Best Pract Res Clin Gastroenterol. (2013) 27:401–14. 10.1016/j.bpg.2013.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sigterman KE, van Pinxteren B, Bonis PA, Lau J, Numans ME. Short-term treatment with proton pump inhibitors, H2-receptor antagonists and prokinetics for gastro-oesophageal reflux disease-like symptoms and endoscopy negative reflux disease. Cochrane Database Syst Rev. (2013) 2013:CD002095. 10.1002/14651858.CD002095.pub5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sifrim D, Zerbib F. Diagnosis and management of patients with reflux symptoms refractory to proton pump inhibitors. Gut. (2012) 61:1340–54. 10.1136/gutjnl-2011-301897 [DOI] [PubMed] [Google Scholar]
- 6.Savarino V, Dulbecco P, de Bortoli N, Ottonello A, Savarino E. The appropriate use of proton pump inhibitors (PPIs): need for a reappraisal. Eur J Intern Med. (2017) 37:19–24. 10.1016/j.ejim.2016.10.007 [DOI] [PubMed] [Google Scholar]
- 7.Dellon ES, Shaheen NJ. Persistent reflux symptoms in the proton pump inhibitor era: the changing face of gastroesophageal reflux disease. Gastroenterology. (2010) 139:7–13. 10.1053/j.gastro.2010.05.016 [DOI] [PubMed] [Google Scholar]
- 8.Kunsch S, Neesse A, Linhart T, Nell C, Gress TM, Ellenrieder V. Prospective evaluation of duodenogastroesophageal reflux in gastroesophageal reflux disease patients refractory to proton pump inhibitor therapy. Digestion. (2012) 86:315–22. 10.1159/000342234 [DOI] [PubMed] [Google Scholar]
- 9.Zerbib F, Sifrim D, Tutuian R, Attwood S, Lundell L. Modern medical and surgical management of difficult-to-treat GORD. United European Gastroenterol J. (2013) 1:21–31. 10.1177/2050640612473964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frazzoni M, Conigliaro R, Mirante VG, Melotti G. The added value of quantitative analysis of on-therapy impedance-pH parameters in distinguishing refractory non-erosive reflux disease from functional heartburn. Neurogastroenterol Motil. (2012) 24:141–6.e87. 10.1111/j.1365-2982.2011.01800.x [DOI] [PubMed] [Google Scholar]
- 11.Zerbib F, Bredenoord AJ, Fass R, Kahrilas PJ, Roman S, Savarino E, et al. ESNM/ANMS consensus paper: diagnosis and management of refractory gastro-esophageal reflux disease. Neurogastroenterol Motil. (2021) 33:e14075. 10.1111/nmo.14075 [DOI] [PubMed] [Google Scholar]
- 12.Yadlapati R, DeLay K. Proton pump inhibitor– refractory gastroesophageal reflux disease. Med Clin N Am. (2019) 103:15–27. 10.1016/j.mcna.2018.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roman S, Gyawali CP, Savarino E, Yadlapati R, Zerbib F, Wu J, et al. GERD consensus group. Ambulatory reflux monitoring for diagnosis of gastro-esophageal reflux disease: Update of the Porto consensus and recommendations from an international consensus group. Neurogastroenterol Motil. (2017) 29:1–15. 10.1111/nmo.13067 [DOI] [PubMed] [Google Scholar]
- 14.Yadlapati R, Vaezi MF, Vela MF, Spechler SJ, Shaheen NJ, Richter J, et al. Management options for patients with GERD and persistent symptoms on proton pump inhibitors: recommendations from an expert panel. Am J Gastroenterol. (2018) 113:980–6. 10.1038/s41395-018-0045-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fass R, Sifrim D. Management of heartburn not responding to proton pump inhibitors. Gut. (2009) 58:295–309. 10.1136/gut.2007.145581 [DOI] [PubMed] [Google Scholar]
- 16.Moraes-Filho JP, Navarro-Rodriguez T, Eisig JN, Barbuti RC, Chinzon D, Quigley EM. Comorbidities are frequent in patients with gastroesophageal reflux disease in a tertiary health care hospital. Clinics. (2009) 64:785–90. 10.1590/S1807-59322009000800013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zerbib F, Belhocine K, Simon M, Capdepont M, Mion F, Bruley des Varannes S, et al. Clinical, but not oesophageal pH-impedance, profiles predict response to proton pump inhibitors in gastro-oesophageal reflux disease. Gut. (2012) 61:501–6. 10.1136/gutjnl-2011-300798 [DOI] [PubMed] [Google Scholar]
- 18.de Bortoli N, Frazzoni L, Savarino EV, Frazzoni M, Martinucci I, Jania A, et al. Functional heartburn overlaps with irritable bowel syndrome more often than GERD. Am J Gastroenterol. (2016) 111:1711–7. 10.1038/ajg.2016.432 [DOI] [PubMed] [Google Scholar]
- 19.Savarino E, Pohl D, Zentilin P, Dulbecco P, Sammito G, Sconfienza L, et al. Functional heartburn has more in common with functional dyspepsia than with non-erosive reflux disease. Gut. (2009) 58:1185–91. 10.1136/gut.2008.175810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Savarino V, Savarino E, Parodi A, Dulbecco P. Functional heartburn and non-erosive reflux disease. Dig Dis. (2007) 25:172–4. 10.1159/000103879 [DOI] [PubMed] [Google Scholar]
- 21.Azer SA, Reddivari AKR. Reflux Esophagitis. Treasure Island, FL: StatPearls Publishing; (2021). [Google Scholar]
- 22.de Bortoli N, Penagini R, Savarino E, Marchi S. Eosinophilic esophagitis: update in diagnosis and management. Position paper by the Italian Society of Gastroenterology and Gastrointestinal Endoscopy (SIGE). Dig Liver Dis. (2017) 49:254–60. 10.1016/j.dld.2016.11.012 [DOI] [PubMed] [Google Scholar]
- 23.Lenti MV, Savarino E, Mauro A, Penagini R, Racca F, Ghisa M, et al. Diagnostic delay and misdiagnosis in eosinophilic oesophagitis. Dig Liver Dis. (2021) 8:S1590-8658(21)00268-1. 10.1016/j.dld.2021.05.017 [DOI] [PubMed] [Google Scholar]
- 24.Kahrilas PJ, Keefer L, Pandolfino JE. Patients with refractory reflux symptoms: what do they have and how should they be managed? Neurogastroenterol Motil. (2015) 27:1195–201. 10.1111/nmo.12644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ates F, Francis DO, Vaezi MF. Refractory gastroesophageal reflux disease: advances and treatment. Expert Rev Gastroenterol Hepatol. (2014) 8:657–67. 10.1586/17474124.2014.910454 [DOI] [PubMed] [Google Scholar]
- 26.Yadlapati R, Kahrilas PJ, Fox MR, Bredenoord AJ, Prakash Gyawali C, Roman S, et al. Esophageal motility disorders on high-resolution manometry: Chicago classification version 4.0©. Neurogastroenterol Motil. (2021) 33:e14058. 10.1111/nmo.14058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tack J, Pauwels A, Roman S, Savarino E, Smout A. ESNM HRM consensus group. European Society for Neurogastroenterology and Motility (ESNM) recommendations for the use of high- resolution manometry of the esophagus. Neurogastroenterol Motil. (2021) 33:e14043. 10.1111/nmo.14043 [DOI] [PubMed] [Google Scholar]
- 28.de Bortoli N, Gyawali PC, Roman S, Tolone S, Sifrim D, Tutuian R, et al. Hypercontractile esophagus from pathophysiology to management: proceedings of the Pisa symposium. Am J Gastroenterol. (2021) 116:263–73. 10.14309/ajg.0000000000001061 [DOI] [PubMed] [Google Scholar]
- 29.Savarino E, de Bortoli N, Bellini M, Galeazzi F, Ribolsi M, Salvador R, et al. Practice guidelines on the use of esophageal manometry - A GISMAD-SIGE-AIGO medical position statement. Dig Liver Dis. (2016) 48:1124–35. 10.1016/j.dld.2016.06.021 [DOI] [PubMed] [Google Scholar]
- 30.Camilleri M, Parkman HP, Shafi MA, Abell TL, Gerson L. Clinical guideline: management of gastroparesis. Am J Gastroenterol. (2013) 108:18–37. 10.1038/ajg.2012.373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perri F, Bellini M, Portincasa P, Parodi A, Bonazzi P, Marzio L, et al. C-octanoic acid breath test (OBT) with a new test meal (EXPIROGer): toward standardization for testing gastric emptying of solids. Dig Liver Dis. (2010) 42:549–53. 10.1016/j.dld.2010.01.001 [DOI] [PubMed] [Google Scholar]
- 32.Savarino E, Frazzoni M, Marabotto E, Zentilin P, Iovino P, Costantini M, et al. A SIGE- SINGEM-AIGO technical review on the clinical use of esophageal reflux monitoring. Dig Liver Dis. (2020) 52:966–80. 10.1016/j.dld.2020.04.031 [DOI] [PubMed] [Google Scholar]
- 33.Savarino E, Bredenoord AJ, Fox M, Pandolfino JE, Roman S, Gyawali CP. International Working Group for Disorders of Gastrointestinal Motility Function. Expert consensus document: advances in the physiological assessment diagnosis of GERD. Nat Rev Gastroenterol Hepatol. (2017) 14:665–76. 10.1038/nrgastro.2017.130 [DOI] [PubMed] [Google Scholar]
- 34.Sifrim D, Holloway R, Silny J, Xin Z, Tack J, Lerut A, et al. Acid, nonacid, and gas reflux in patients with gastroesophageal reflux disease during ambulatory 24 -hour pH-impedance recordings. Gastroenterology. (2001) 120:1588–98. 10.1053/gast.2001.24841 [DOI] [PubMed] [Google Scholar]
- 35.Scarpellini E, Ang D, Pauwels A, De Santis A, Vanuytsel T, Tack J. Management of refractory typical GERD symptoms. Nat Rev Gastroenterol Hepatol. (2016) 13:281–94. 10.1038/nrgastro.2016.50 [DOI] [PubMed] [Google Scholar]
- 36.Zerbib F, Roman S, Ropert A, des Varannes SB, Pouderoux P, Chaput U. Esophageal pH- impedance monitoring and symptom analysis in GERD: a study in patients off and on therapy. Am J Gastroenterol. (2006) 101:1956–63. 10.1111/j.1572-0241.2006.00711.x [DOI] [PubMed] [Google Scholar]
- 37.Gyawali CP, Kahrilas PJ, Savarino E, Zerbib F, Mion F, Smout AJPM, et al. Modern diagnosis of GERD: the Lyon Consensus. Gut. (2018) 67:1351–62. 10.1136/gutjnl-2017-314722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Savarino E, Tutuian R, Zentilin P, Dulbecco P, Pohl D, Marabotto E, et al. Characteristics of reflux episodes and symptom association in patients with erosive esophagitis and non-erosive reflux disease: study using combined impedance-pH off therapy. Am J Gastroenterol. (2010) 105:1053–61. 10.1038/ajg.2009.670 [DOI] [PubMed] [Google Scholar]
- 39.Frazzoni M, Frazzoni L, Tolone S, De Bortoli N, Savarino V, Savarino E. Lack of improvement of impaired chemical clearance characterizes PPI-refractory reflux-related heartburn. Am J Gastroenterol. (2018) 113:670–6. 10.1038/s41395-018-0044-5 [DOI] [PubMed] [Google Scholar]
- 40.Frazzoni M, Savarino E, de Bortoli N, Martinucci I, Furnari M, Frazzoni L, et al. Analyses of the post-reflux swallow-induced peristaltic wave index and nocturnal baseline impedance parameters increase the diagnostic yield of impedance-pH monitoring of patients with reflux disease. Clin Gastroenterol Hepatol. (2016) 14:40–6. 10.1016/j.cgh.2015.06.026 [DOI] [PubMed] [Google Scholar]
- 41.de Bortoli N, Martinucci I, Savarino E, Tutuian R, Frazzoni M, Piaggi P, et al. Association between baseline impedance values and response proton pump inhibitors in patients with heartburn. Clin Gastroenterol Hepatol. (2015) 13:1082–8.e1. 10.1016/j.cgh.2014.11.035 [DOI] [PubMed] [Google Scholar]
- 42.Savarino V, Marabotto E, Zentilin P, Demarzo MG, Pellegatta G, Frazzoni M, et al. Esophageal reflux hypersensitivity: Non-GERD or still GERD? Dig Liver Dis. (2020) 52:1413–20. 10.1016/j.dld.2020.10.003 [DOI] [PubMed] [Google Scholar]
- 43.Frazzoni M, de Bortoli N, Frazzoni L, Tolone S, Furnari M, Martinucci I, et al. The added diagnostic value of postreflux swallow-induced peristaltic wave index and nocturnal baseline impedance in refractory reflux disease studied with on-therapy impedance-pH monitoring. Neurogastroenterol Motil. (2017) 48:1124–35. 10.1111/nmo.12947 [DOI] [PubMed] [Google Scholar]
- 44.Spechler SJ, Hunter JG, Jones KM, Lee R, Smith BR, Mashimo H, et al. Randomized trial of medical versus surgical treatment for refractory heartburn. N Engl J Med. (2019) 381:1513–23. 10.1056/NEJMoa1811424 [DOI] [PubMed] [Google Scholar]
- 45.Dominigues G, Moraes-Filho JPP. Noncompliance is an impact factor in the treatment of gastroesophageal reflux disease. Expert Rev Gastroenterol Hepatol. (2014) 8:761–5. 10.1586/17474124.2014.911660 [DOI] [PubMed] [Google Scholar]
- 46.Gunaratnam NT, Jessup TP, Inadomi J, Lascewski DP. Sub-optimal proton pump inhibitor dosing is prevalent in patients with poorly controlled gastro-oesophageal reflux disease. Aliment Pharmacol Ther. (2006) 23:1473–7. 10.1111/j.1365-2036.2006.02911.x [DOI] [PubMed] [Google Scholar]
- 47.Van Soest EM, Siersema PD, Dieleman JP, Sturkenboom MC, Kuipers EJ. Persistence and adherence to proton pump inhibitors in daily clinical practice. Aliment Pharmacol Ther. (2006) 24:377–85. 10.1111/j.1365-2036.2006.02982.x [DOI] [PubMed] [Google Scholar]
- 48.Gosselin A, Luo R, Lohoues H, Toy E, Lewis B, Crawley J, et al. The impact of proton pump inhibitor compliance on health- care resource utilization and costs in patients with gastroesophageal reflux disease. Value Health. (2009) 12:34–9. 10.1111/j.1524-4733.2008.00399.x [DOI] [PubMed] [Google Scholar]
- 49.Fass R, Thomas S, Traxler B, Sostek M. Patient reported outcome of heartburn improvement: doubling the proton pump inhibitor (PPI) dose in patients who failed standard dose PPI vs. switching to a different PPI. Gastroenterology. (2004) 146:A37. [Google Scholar]
- 50.Hungin AP, Rubin G, O'Flanagan H. Factors influencing compliance in long-term proton pump inhibitor therapy in general practice. Br J Gen Pract. (1999) 49:463–4. [PMC free article] [PubMed] [Google Scholar]
- 51.Scarpignato C, Richard H. Hunt. Editorial: acid suppression with potassium-competitive acid blockers—dismissing genotype concerns. Aliment Pharmacol Ther. (2021) 53:187–8. 10.1111/apt.16139 [DOI] [PubMed] [Google Scholar]
- 52.Hagymási K, Müllner K, Herszényi L, Tulassay Z. Update on the pharmacogenomics of proton pump inhibitors. Pharmacogenomics. (2011) 12:873–88. 10.2217/pgs.11.4 [DOI] [PubMed] [Google Scholar]
- 53.Ichikawa H, Sugimoto M, Sugimoto K, Andoh A, Furuta T. Rapid metabolizer genotype of CYP2C19 is a risk factor of being refractory to proton pump inhibitor therapy for reflux esophagitis. J Gastroenterol Hepatol. (2016) 31:716–26. 10.1111/jgh.13233 [DOI] [PubMed] [Google Scholar]
- 54.Chen WY, Chang WL, Tsai YC, Cheng HC, Lu CC, Sheu BS. Double-dosed pantoprazole accelerates the sustained symptomatic response in overweight and obese patients with reflux esophagitis in Los Angeles grades A and B. Am J Gastroenterol. (2010) 105:1046–52. 10.1038/ajg.2009.632 [DOI] [PubMed] [Google Scholar]
- 55.Hillman L, Yadlapati R, Thuluvath AJ, Berendsen MA, Pandolfino JE. A review of medical therapy for proton pump inhibitor nonresponsive gastroesophageal reflux disease. Dis Esophagus. (2017) 30:1–15. 10.1093/dote/dox055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Graham DY, Tansel A. Interchangeable use of proton pump inhibitors based on relative potency. Clin Gastroenterol Hepatol. (2018) 16:800–8. 10.1016/j.cgh.2017.09.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Katz PO, Koch FK, Ballard ED, Bagin RG, Gautille TC, Checani GC, et al. Comparison of the effects of immediate-release omeprazole oral suspension, delayed-release lansoprazole capsules and delayed-release esomeprazole capsules on nocturnal gastric acidity after bedtime dosing in patients with night-time GERD symptoms. Aliment Pharmacol Ther. (2007) 25:197–205. 10.1111/j.1365-2036.2006.03191.x [DOI] [PubMed] [Google Scholar]
- 58.Ang D, Hussain I, Fock KM. Tu1885 understanding the cause of persistent GERD symptoms despite proton pump inhibitor therapy:impedance-pH monitoring revisited. Gastroenterology. (2014) 146:863–4. 10.1016/S0016-5085(14)63140-1 [DOI] [Google Scholar]
- 59.Frazzoni M, Conigliaro R, Melotti G. Weakly acidic refluxes have a major role in the pathogenesis of proton pump inhibitor-resistant reflux oesophagitis. Aliment Pharmacol Ther. (2011) 33:601–6. 10.1111/j.1365-2036.2010.04550.x [DOI] [PubMed] [Google Scholar]
- 60.Kohata Y, Fujiwara Y, Machida H, Okazaki H, Yamagami H, Tanigawa T, et al. Pathogenesis of proton-pump inhibitor-refractory non-erosive reflux disease according to multichannel intraluminal impedance-pH monitoring. J Gastroenterol Hepatol. (2012) 27 (Suppl. 3):58–62. 10.1111/j.1440-1746.2012.07074.x [DOI] [PubMed] [Google Scholar]
- 61.Pearson JP, Parikh S, Orlando RC, Johnston N, Allen J, Tinling SP, et al. Review article: reflux and its consequences–the laryngeal, pulmonary and oesophageal manifestations. Conference held in conjunction with the 9th International Symposium on Human Pepsin (ISHP) Kingston-upon-Hull, UK, 21-23 April 2010. Aliment Pharmacol Ther. (2011) 33 (Suppl. 1):1 −71. 10.1111/j.1365-2036.2011.04581.x [DOI] [PubMed] [Google Scholar]
- 62.de Bortoli N, Martinucci I, Savarino E, Franchi R, Bertani L, Russo S, et al. Lower pH values of weakly acidic refluxes as determinants of heartburn perception in gastroesophageal reflux disease patients with normal esophageal acid exposure. Dis Esophagus. (2016) 29:3–9. 10.1111/dote.12284 [DOI] [PubMed] [Google Scholar]
- 63.de Bortoli N, Ottonello A, Zerbib F, Sifrim D, Gyawali CP, Savarino E. Between GERD and NERD: the relevance of weakly acidic reflux. Ann N Y Acad Sci. (2016) 1380:218–29. 10.1111/nyas.13169 [DOI] [PubMed] [Google Scholar]
- 64.Emerenziani S, Ribolsi M, Guarino MP, Balestrieri P, Altomare A, Rescio MP, et al. Acid reflux episodes sensitize the esophagus to perception of weakly acidic and mixed reflux in non-erosive reflux disease patients. Neurogastroenterol Motil. (2014) 26:108–14. 10.1111/nmo.12239 [DOI] [PubMed] [Google Scholar]
- 65.Rohof WO, Bennink RJ, de Jonge H, Boeckxstaens GE. Increased proximal reflux in a hypersensitive esophagus might explain symptoms resistant to proton pump inhibitors in patients with gastroesophageal reflux disease. Clin Gastroenterol Hepatol. (2014) 12:1647–55. 10.1016/j.cgh.2013.10.026 [DOI] [PubMed] [Google Scholar]
- 66.Zheng G, Wu SP, Hu Y, Smith DE, Wiley JW, Hong S. Corticosterone mediates stress-related increased intestinal permeability in a region-specific manner. Neurogastroenterol Motil. (2013) 25:e127–39. 10.1111/nmo.12066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xu D, Gao J, Gillilland M, 3rd, Wu X, Song I, Kao JY, et al. Rifaximin alters intestinal bacteria and prevents stress-induced gut inflammation and visceral hyperalgesia in rats. Gastroenterology. (2014) 146:484–96.e4. 10.1053/j.gastro.2013.10.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.de Bortoli N, Gyawali CP, Frazzoni M, Tolone S, Frazzoni L, Vichi E, et al. Bile reflux in patients with nerd is associated with more severe heartburn and lower values of mean nocturnal baseline impedance and chemical clearance. Neurogastroenterol Motil. (2020) 32:e13919. 10.1111/nmo.13919 [DOI] [PubMed] [Google Scholar]
- 69.Gasiorowska A, Navarro-Rodriguez T, Wendel C, Krupinski E, Perry ZH, Koenig K, et al. Comparison of the degree of duodenogastroesophageal reflux and acid reflux between patients who failed to respond and those who were successfully treated with a proton pump inhibitor once daily. Am J Gastroenterol. (2009) 104:2005–13. 10.1038/ajg.2009.240 [DOI] [PubMed] [Google Scholar]
- 70.Herregods TV, Bredenoord AJ, Smout AJ. Pathophysiology of gastroesophageal reflux disease: new understanding in a new era. Neurogastroenterol Motil. (2015) 27:1202–13. 10.1111/nmo.12611 [DOI] [PubMed] [Google Scholar]
- 71.Tolone S, De Bortoli N, Marabotto E, de Cassan C, Bodini G, Roman S, et al. Esophagogastric junction contractility for clinical assessment in patients with GERD: a real added value? Neurogastroenterol Motil. (2015) 27:1423–31. 10.1111/nmo.12638 [DOI] [PubMed] [Google Scholar]
- 72.Tolone S, de Cassan C, de Bortoli N, Roman S, Galeazzi F, Salvador R, et al. Esophagogastric junction morphology is associated with a positive impedance-pH monitoring in patients with GERD. Neurogastroenterol Motil. (2015) 27:1175–82. 10.1111/nmo.12606 [DOI] [PubMed] [Google Scholar]
- 73.Ribolsi M, Savarino E, Rogers B, Rengarajan A, Coletta MD, Ghisa M, et al. High-resolution manometry determinants of refractoriness of reflux symptoms to proton pump inhibitor therapy. J Neurogastroenterol Motil. (2020) 26:447–54. 10.5056/jnm19153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Martinucci I, Savarino EV, Pandolfino JE, Russo S, Bellini M, Tolone S, et al. Vigor of peristalsis during multiple rapid swallows is inversely correlated with acid exposure time in patients with NERD. Neurogastroenterol Motil. (2016) 28:243–50. 10.1111/nmo.12719 [DOI] [PubMed] [Google Scholar]
- 75.Savarino E, Gemignani L, Pohl D, Zentilin P, Dulbecco P, Assandri L, et al. Oesophageal motility and bolus transit abnormalities increase in parallel with the severity of gastro-oesophageal reflux disease. Aliment Pharmacol Ther. (2011) 34:476–86. 10.1111/j.1365-2036.2011.04742.x [DOI] [PubMed] [Google Scholar]
- 76.Rogers BD, Rengarajan A, Ribolsi M, Ghisa M, Quader F, Penagini R, et al. Postreflux swallow- induced peristaltic wave index from pH-impedance monitoring associates with esophageal body motility and esophageal acid burden. Neurogastroenterol Motil. (2021) 33:e13973. 10.1111/nmo.13973 [DOI] [PubMed] [Google Scholar]
- 77.Ribolsi M, Gyawali CP, Savarino E, Rogers B, Rengarajan A, Della Coletta M, et al. Correlation between reflux burden, peristaltic function, and mucosal integrity in GERD patients. Neurogastroenterol Motil. (2020) 32:e13752. 10.1111/nmo.13752 [DOI] [PubMed] [Google Scholar]
- 78.Tack J, Pandolfino JE. Pathophysiology of gastroesophageal reflux disease. Gastroenterology. (2018) 154:277–88. 10.1053/j.gastro.2017.09.047 [DOI] [PubMed] [Google Scholar]
- 79.Zentilin P, Dulbecco P, Savarino E, Parodi A, Iiritano E, Bilardi C, et al. An evaluation of the antireflux properties of sodium alginate by means of combined multichannel intraluminal impedance and pH-metry. Aliment Pharmacol Ther. (2005) 21:29–34. 10.1111/j.1365-2036.2004.02298.x [DOI] [PubMed] [Google Scholar]
- 80.Mermelstein J, Mermelstein AC, Chait MM. Proton pump inhibitor- refractory gastroesophageal reflux disease: challenges and solutions. Clin Exp Gastroenterol. (2018) 11:119–34. 10.2147/CEG.S121056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Emerenziani S, Sifrim D. Gastroesophageal reflux and gastric emptying, revisited. Curr Gastroenterol Rep. (2005) 7:190–5. 10.1007/s11894-005-0033-x [DOI] [PubMed] [Google Scholar]
- 82.Kessing BF, Smout AJ, Bennink RJ, Kraaijpoel N, Oors JM, Bredenoord AJ. Prucalopride decreases esophageal acid exposure and accelerates gastric emptying in healthy subjects. Neurogastroenterol Motil. (2014) 26:1079–86. 10.1111/nmo.12359 [DOI] [PubMed] [Google Scholar]
- 83.Tack J. Is there a unifying role for visceral hypersensitivity and irritable bowel syndrome in non- erosive reflux disease? Digestion. (2008) 78 (Suppl. 1):42–5. 10.1159/000151254 [DOI] [PubMed] [Google Scholar]
- 84.Boeckxstaens V, Pauwels A, Blondeau K, Tack J. Refractory GERD patients display increased visceral hypersensitivity for thermal, chemical and mechanical esophageal stimulation. Gastroenterology. (2013) 133:S936. 10.1016/S0016-5085(13)63480-0 [DOI] [Google Scholar]
- 85.Keefer L, Eident K, Kahrilas I, Roman S, Pandolfino JE. Symptom reporting among PPI non responders may be driven by esophageal hypervigilance and not reflux phenotype. Gastroenterology. (2015) 148:S-147. 10.1016/S0016-5085(15)30500-X [DOI] [Google Scholar]
- 86.Boeckxstaens G, El-Serag HB, Smout AJ, Kahrilas PJ. Symptomatic reflux disease: the present, the past and the future. Gut. (2014) 63:1185–93. 10.1136/gutjnl-2013-306393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Katz PO, Gerson LB, Vela MF. Guidelines for the diagnosis and management of gastroesophageal reflux disease. Am J Gastroenterol. (2013) 108:308–28. 10.1038/ajg.2012.444 [DOI] [PubMed] [Google Scholar]
- 88.de Bortoli N, Guidi G, Martinucci I, Savarino E, Imam H, Bertani L, et al. Voluntary and controlled weight loss can reduce symptoms and proton pump inhibitor use and dosage in patients with gastroesophageal reflux disease: a comparative study. Dis Esophagus. (2016) 29:197–204. 10.1111/dote.12319 [DOI] [PubMed] [Google Scholar]
- 89.McDougall NI, Johnston BT, Collins JS, McFarland RJ, Love AH. Three- to 4.5- year prospective study of prognostic indicators in gastro-oesophageal reflux disease. Scand J Gastroenterol. (1998) 33:1016–22. 10.1080/003655298750026688 [DOI] [PubMed] [Google Scholar]
- 90.Nilsson M, Johnsen R, Ye W, Hveem K, Lagergren J. Lifestyle related risk factors in the aetiology of gastro- oesophageal reflux. Gut. (2004) 59:1730–5. 10.1136/gut.2004.043265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Morozov S, Isakov V, Konovalova M. Fiber-enriched diet helps to control symptoms and improves esophageal motility in patients with non- erosive gastroesophageal reflux disease. World J Gastroenterol. (2018) 24:2291–9. 10.3748/wjg.v24.i21.2291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Martinucci I, Guidi G, Savarino EV, Frazzoni M, Tolone S, Frazzoni L, et al. Vegetal and animal food proteins have a different impact in the first postprandial hour of impedance-ph analysis in patients with heartburn. Gastroenterol Res Pract. (2018) 2018:7572430. 10.1155/2018/7572430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kahrilas PJ, Shaheen NJ, Vaezi M, collaboration with the AGAI . Medical Position Panel on GERD management. American Gastroenterological Association Institute medical position statement: management of gastroesophageal reflux disease. Gastroenterology. (2008) 135:1383–91. 10.1053/j.gastro.2008.08.045 [DOI] [PubMed] [Google Scholar]
- 94.Tosetti C, Savarino E, Benedetto E, De Bastiani R. Study group for the evaluation of GERD triggering foods. elimination of dietary triggers is successful in treating symptoms of gastroesophageal reflux disease. Dig Dis Sci. (2021) 66:1565–71. 10.1007/s10620-020-06414-z [DOI] [PubMed] [Google Scholar]
- 95.Monnikes H, Heading RC, Schmitt H, Doerfler H. Influence of irritable bowel syndrome on treatment outcome in gastroesophageal reflux disease. World J Gastroenterol. (2011) 17:3235–41. 10.3748/wjg.v17.i27.3235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rivière P, Vauquelin B, Rolland E, Melchior C, Roman S, Bruley des Varannes S, et al. Low FODMAPs diet or usual dietary advice for the treatment of refractory gastroesophageal reflux disease: an open-labeled randomized trial. Neurogastroenterol Motil. (2021) 33:e14181. 10.1111/nmo.14181 [DOI] [PubMed] [Google Scholar]
- 97.Eusebi LH, Ratnakumaran R, Yuan Y, Solaymani-Dodaran M, Bazzoli F, Ford AC. Global prevalence of, and risk factors for, gastro-oesophageal reflux symptoms: a meta-analysis. Gut. (2018) 67:430–40. 10.1136/gutjnl-2016-313589 [DOI] [PubMed] [Google Scholar]
- 98.Vaezi MF, Fass R, Vakil N, Reasner DS, Mittleman RS, Hall M, et al. IW-3718 reduces heartburn severity in patients with refractory gastroesophageal reflux disease in a trial. Gastroenterology. (2020) 158:2093–103. 10.1053/j.gastro.2020.02.031 [DOI] [PubMed] [Google Scholar]
- 99.Dal-Paz K, Moraes-Filho JP, Navarro-Rodriguez T, Eisig JN K, Barbuti R, Quigley EMM. Low levels of adherence with proton pump inhibitor therapy contribute to therapeutic failure in gastroesophageal reflux disease. Dis Esophagus. (2012) 25:107–13. 10.1111/j.1442-2050.2011.01227.x [DOI] [PubMed] [Google Scholar]
- 100.Fass R, Sontag SJ, Traxler B, Sostek M. Tratment of patients with persistent heartburn symptoms: a double- blind, trial. Clin GastroenterolHepatol. (2006) 4:50–6. 10.1016/S1542-3565(05)00860-8 [DOI] [PubMed] [Google Scholar]
- 101.Abraham NS. Proton pump inhibitors: potential adverse effects. Current Opin Gastroenterol. (2012) 28:615–20. 10.1097/MOG.0b013e328358d5b9 [DOI] [PubMed] [Google Scholar]
- 102.Mermelstein J, Mermelstein AC, Chait MM. Proton pump inhibitors for the treatment of patients with erosive esophagitis and gastroesophageal reflux disease: current evidence and safety of dexlansoprazole. Clin Exp Gastroenterol. (2016) 9:163–72. 10.2147/CEG.S91602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Fass R, Frazier R. The role of dexlansoprazole modified-release in the management of gastroesophageal reflux disease. Therap Adv Gastroenterol. (2017) 10:243–51. 10.1177/1756283X16681701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hatlebakk JG, Katz PO, Kuo B, Castell DO. Nocturnal gastric acidity and acid breakthrough on different regimens of omeprazole 40 mg daily. Aliment Pharmacol. Ther. (1998) 12:1235–40. 10.1046/j.1365-2036.1998.00426.x [DOI] [PubMed] [Google Scholar]
- 105.Moennikes H, Weber S, Tebbe J, Luhmann R, Fischer R, Arnold R, et al. Comparison of pantoprazole 20 mg twice daily with 40 mg once daily on intragastric pH in healthy volunteers. Gastroenterology. (1998) 114:A232. 10.1016/S0016-5085(98)80942-6 [DOI] [Google Scholar]
- 106.Hammer J, Schmidt B. Effect of splitting the dose of esomeprazole on gastric acidity and nocturnal acid breakthrough. Aliment Pharmacol Ther. (2004) 19:1105–110. 10.1111/j.1365-2036.2004.01949.x [DOI] [PubMed] [Google Scholar]
- 107.Fass R, Murthy U, Hayden CW, Malagon IB, Pulliam G, Wendel C, et al. Omeprazole 40 mg once a day is equally effective as lansoprazole 30 mg twice a day in symptom control of patients with gastro-oesophageal reflux disease (GERD) who are resistant to conventional- dose lansoprazole therapy- a prospective, multi- centre study. Aliment Pharmacol Ther. (2000) 14:1959–603. 10.1046/j.1365-2036.2000.00882.x [DOI] [PubMed] [Google Scholar]
- 108.Klok RM, Postma MJ, van Hout BA, Brouwers JR. Meta- analysis: comparing the efficacy of proton pump inhibitors in short- term use. Aliment Pharmacol Ther. (2003) 17:1237–45. 10.1046/j.1365-2036.2003.01562.x [DOI] [PubMed] [Google Scholar]
- 109.Armstrong D, Marshall JK, Chiba N, Enns R, Fallone CA, Fass R, et al. Canadian Consensus Conference on the management of gastroesophageal reflux disease in adults - update 2004. Can J Gastroenterol. (2005) 19:15–35. 10.1155/2005/836030 [DOI] [PubMed] [Google Scholar]
- 110.World Health Organization Collaborating Centre for Drug Statistics Methodology . Available online at: http://www.whocc.no/atc_ddd_index/?code1/4A02BC&showdescription1/4yes (accessed March 14, 2021).
- 111.Grime J, Pollock K, Blenkinsopp A. Proton pump inhibitors: perspectives of patients and their GPs. Br J Gen Pract. (2001) 51:703–11. [PMC free article] [PubMed] [Google Scholar]
- 112.Sagar M, Tybring G, Dahl ML, Bertilsson L, Seensalu R. Effects of omeprazole on intragastric pH and plasma gastrin are dependent on the CYP2C19 polymorphism. Gastroenterology. (2009) 119:670–6. 10.1053/gast.2000.16515 [DOI] [PubMed] [Google Scholar]
- 113.Furuta T, Sugimoto M, Shirai N, Ishizaki T. CYP2C19 pharmacogenomics associated with therapy of Helicobacter pylori infection and gastro-esophageal reflux diseases with a proton pump inhibitor. Pharmacogenomics. (2007) 8:1199–210. 10.2217/14622416.8.9.1199 [DOI] [PubMed] [Google Scholar]
- 114.Hungin APS, Molloy-Bland M, Scarpignato C. Revisiting montreal: new insights into symptoms and their causes, and implications for the future of GERD. Am J Gastroenterol. (2019) 114:414–21. 10.1038/s41395-018-0287-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Boeckxstaens GE, Smout A. Systematic review: role of acid, weakly acidic and weakly alkaline reflux in gastro- oesphoageal reflux disease. Aliment Pharmacol Ther. (2010) 32:334–43. 10.1111/j.1365-2036.2010.04358.x [DOI] [PubMed] [Google Scholar]
- 116.Marrero JM, de Caestecker JS, Maxwell JD. Effect of famotidine on oesophageal sensitivity in gastro- oesophageal reflux disease. Gut. (1994) 95:447–50. 10.1136/gut.35.4.447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Mainie I, Tutuian R, Castell DO. Addition of a H2 receptor antagonist to PPI improves acid control and decreases nocturnal acid breakthrough. J. Clin. Gastroenterol. (2008) 42:676–9. 10.1097/MCG.0b013e31814a4e5c [DOI] [PubMed] [Google Scholar]
- 118.Fackler WK, Ours TM, Vaezi MF, Richter JE. Long-term effect of H2RA therapy on nocturnal gastric acid breakthrough. Gastroenterology. (2002) 122:625–32. 10.1053/gast.2002.31876 [DOI] [PubMed] [Google Scholar]
- 119.Rackoff A, Agrawal A, Hila A, Mainie I, Tutuian R, Castell DO. Histamine-2 receptor antagonists at night improve gastroesophageal reflux disease symptoms for patients on proton pump inhibitor therapy. Dis Esophagus. (2005) 18:370–3. 10.1111/j.1442-2050.2005.00518.x [DOI] [PubMed] [Google Scholar]
- 120.Abdul-Hussein M, Freeman J, Castell D. Concomitant administration of a histamine 2 receptor antagonist and proton pump inhibitor enhances gastric acid suppression. Pharmacotherapy. (2015) 35:1124–9. 10.1002/phar.1665 [DOI] [PubMed] [Google Scholar]
- 121.Reimer C, Lødrup AB, Smith G, Wilkinson J, Bytzer P. Randomised clinical trial: alginate (Gaviscon Advance) vs. placebo as add-on therapy in reflux patients with inadequate response to a once daily proton pump inhibitor. Aliment Pharmacol Ther. (2016) 43:899–909. 10.1111/apt.13567 [DOI] [PubMed] [Google Scholar]
- 122.Hershcovici T, Fass R. Pharmacological management of GERD: where does it stand now? Trends Pharmacol Sci. (2011) 32:258–64. 10.1016/j.tips.2011.02.007 [DOI] [PubMed] [Google Scholar]
- 123.Nennstiel S, Bajbouj M, Schmid RM Becker V. Prucalopride reduces the number of reflux episodes and improves subjective symptoms in gastroesophageal reflux disease: a case series. J Med Case Rep. (2014) 8:34. 10.1186/1752-1947-8-34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Richter J. How to manage refractory GERD. Nat Clin Pract Gastroenterol Hepatol. (2007) 4:658–64. 10.1038/ncpgasthep0979 [DOI] [PubMed] [Google Scholar]
- 125.Savarino V, Marabotto E, Zentilin P, Demarzo MG, de Bortoli N, Savarino E. Pharmacological management of gastro-esophageal reflux disease: an update of the state-of-the-art. Drug Des Devel Ther. (2021) 15:1609–21. 10.2147/DDDT.S306371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Savarino V, Pace F, Scarpignato C. Randomised clinical trial: mucosal protection combined with acid suppression in the treatment of nonerosive reflux disease- efficacy of Esoxx, a hyaluronic acid- chondroitin sulphate based bioadhesive formulation. Aliment Pharmacol Ther. (2017) 45:631–42. 10.1111/apt.13914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Akiyama J, Hosaka H, Kuribayashi S, Moriyasu S, Hisada Y, Okubo H, et al. Efficacy of vonoprazan, a novel potassium-competitive acid blocker, in patients with proton pump inhibitor- refractory acid reflux. Digestion. (2019) 24:1–10. 10.1159/000497775 [DOI] [PubMed] [Google Scholar]
- 128.Graham DY, Dore MP. Update on the use of vonoprazan: a competitive acid blocker. Gastroenterology. (2018) 154:462–6. 10.1053/j.gastro.2018.01.018 [DOI] [PubMed] [Google Scholar]
- 129.Hamada S, Ihara E, Ikeda H, Muta K, Ogino H, Chinen T, et al. Clinical characterization of vonoprazan- refractory gastroesophageal reflux disease. Digestion. (2019) 102:197–204. 10.1159/000503340 [DOI] [PubMed] [Google Scholar]
- 130.Siddiqui A, Rodriguez-Stanley S, Zubaidi S, Miner PB, Jr. Esophageal visceral sensitivity to bile salts in patients with functional heartburn and in healthy control subjects. Dig Dis Sci. (2005) 50:81–5. 10.1007/s10620-005-1282-0 [DOI] [PubMed] [Google Scholar]
- 131.Martinucci I, Blandizzi C, Bodini G, Marabotto E, Savarino V, Marchi S, et al. Vonoprazan fumarate for the management of acid-related diseases. Expert Opin Pharmacother. (2017) 18:1145–52. 10.1080/14656566.2017.1346087 [DOI] [PubMed] [Google Scholar]
- 132.Savarino E, Martinucci I, Furnari M, Romana C, Pellegatta G, Moscatelli A, et al. Vonoprazan for treatment of gastroesophageal reflux: pharmacodynamic and pharmacokinetic considerations. Expert Opin Drug Metab Toxicol. (2016) 12:1333–41. 10.1080/17425255.2016.1214714 [DOI] [PubMed] [Google Scholar]
- 133.Roman S, Mion F. Refractory GERD, beyond proton pump inhibitors. Current opin Pharmacol. (2018) 43:99–103. 10.1016/j.coph.2018.09.001 [DOI] [PubMed] [Google Scholar]
- 134.Shinozaki S, Osawa H, Hayashi Y, Sakamoto H, Kobayashi Y, Kawarai Lefor A, et al. Vonoprazan 10 mg daily is effective for the treatment of patients with proton pump inhibitor- resistant gastroesophageal reflux disease. Biomed Rep. (2017) 7:231–5. 10.3892/br.2017.947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Shinozaki S, Osawa H, Hayashi Y, Miura Y, Lefor AK, Yamamoto H. Long-term vonoprazan therapy is effective for controlling symptomatic proton pump inhibitor-resistant gastroesophageal reflux disease. Biomed Rep. (2021) 14:32. 10.3892/br.2021.1408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Okuyama M, Nakahara K, Iwakura N, Hasegawa T, Oyama M, Inoue A, et al. Factors associated with potassium- competitive acid blocker non- response in patients with proton pump inhibitor- refractory gastroesophageal reflux disease. Digestion. (2017) 95:281–7. 10.1159/000475658 [DOI] [PubMed] [Google Scholar]
- 137.Herszényi L, Bakucz T, Barabás L, Tulassay Z. Pharmacological approach to gastric acid suppression: past, present, and future. Dig Dis. (2020) 38 (suppl. 2):104–11. 10.1159/000505204 [DOI] [PubMed] [Google Scholar]
- 138.Usai-Satta P, Bellini M, Morelli O, Geri F, Lai M, Bassotti G. Gastroparesis: new insights into an old disease. World J Gastroenterol. (2020) 26:2333–48. 10.3748/wjg.v26.i19.2333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ren LH, Chen WX, Qian LJ, Li S, Gu M, Shi RH. Addition of prokinetics to PPI therapy in gastroesophageal reflux disease: a meta- analysis. World J Gastroenterol. (2014) 20:2412–9. 10.3748/wjg.v20.i9.2412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Richter JE, Sabesin SM, Kogut DG, Kerr RM, Wruble LD, Collen MJ. Omeprazole versus ranitidine or ranitidine/ metoclopramide in poorly responsive symptomatica gastroesophageal reflux disease. Am J Gastroenterol. (1996) 91:1766–72. [PubMed] [Google Scholar]
- 141.Bron B, Massih L. Domperidone: a drug with powerful action on the lower esophageal sphincter pressure. Digestion. (1980) 20:375–8. 10.1159/000198476 [DOI] [PubMed] [Google Scholar]
- 142.Taghvaei T, Kazemi A, Hosseini V, Hamidian M, Tirgar Fakheri H, Hashemi SA, et al. Evaluation of the additive effect of domperidone on patients with refractory gastroesophageal reflux disease; a double blind clinical trial. Middle East J Dig Dis. (2019) 11:24–31. 10.15171/mejdd.2018.124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Tack J, Camilleri M, Chang L, Chey WD, Galligan JJ, Lacy BE, et al. Systematic review: cardiovascular safety profile of 5-HT4 agonists developed for gastrointestinal disorders. Aliment Pharmacol Ther. (2012) 35:745–67. 10.1111/j.1365-2036.2012.05011.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Futagami S, Iwakiri K, Shindo T, Kawagoe T, Horie A, Shimpuku M, et al. The prokinetic effect of mosapride citrate combined with omeprazole therapy improves clinical symptoms and gastric emptying in PPI-resistant NERD patients with delayed gastric emptying. J Gastroenterol. (2010) 45:413–21. 10.1007/s00535-009-0173-0 [DOI] [PubMed] [Google Scholar]
- 145.Shaheen NJ, Adler J, Dedrie S, Johnson D, Malfertheiner P, Miner P, et al. Randomised clinical trial: the 5 -HT4 agonist revexepride in patients with gastro- oesophageal reflux disease who have persistent symptoms despite PPI therapy. Aliment Pharmacol Ther. (2011) 33:650–61. 10.1111/apt.13115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Koek GH, Sidrim D, Lerut T, Janssens J, Tack J. Effect of the GABAB agonist baclofen in patients with symptoms and duodeno- gastro- oesophageal reflux refractory to proton pump inhibitors. Gut. (2003) 52:1397–402. 10.1136/gut.52.10.1397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Li S, Shi S, Chen F, Lin J. The effects of baclofen for the treatment of gastroesophageal reflux disease: a meta- analysis of controlled trials. Gastroenterol Res Pract. (2014) 2014:307805. 10.1155/2014/307805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Shaheen NJ, Denison H, Björck K, Karlsson M, Sillberg DG. Efficacy and safety of lesogaberan in gastro-oesophageal reflux disease: a - controlled trial. Gut. (2013) 62:1248–55. 10.1136/gutjnl-2012-302737 [DOI] [PubMed] [Google Scholar]
- 149.Lee H, Kim JH, Min BH, Lee JH, Son HJ, Kim JJ, et al. Efficacy of venlafaxine for symptomatic relief in young patients with functional chest pain: a randomized, double- blind, placebo- controlled, crossover trial. Am J Gastroenterol. (2010) 105:1504–12. 10.1038/ajg.2010.82 [DOI] [PubMed] [Google Scholar]
- 150.Rouzade-Dominguez ML, Pezous N, David OJ, Tutuian R, Bruley des Varannes S, Tack J, et al. The selective metabotropic glutamate 5 antagonist mavoglurant (AFQ056) reduces the incidence of reflux episodes in dogs and patients with moderate to severe gastroesophageal reflux disease. Neurogastroenterol Motil. (2017) 29. 10.1111/nmo.13058 [DOI] [PubMed] [Google Scholar]
- 151.Fock KM, Talley N, Goh KL, Sugano K, Sugano K, Katelaris P, et al. Asia- Pacific consensus on the management of gastro- oesophageal reflux disease: an uptodate focusing on refractory reflux disease and Barrett's oesophagus. Gut. (2016) 65:1402–15. 10.1136/gutjnl-2016-311715 [DOI] [PubMed] [Google Scholar]
- 152.Varia I, Logue E, O'Connor C, Newby K, Wagner HR, Davenport C, et al. Randomized trial of sertraline in patients with unexplained chest pain of noncardiac origin. Am Heart J. (2000) 140:367–72. 10.1067/mhj.2000.108514 [DOI] [PubMed] [Google Scholar]
- 153.Hershcovici T, Jha LK, Gadam R, Fass OZ, Wendel C, Navarro-Rodriguez T, et al. Comparison of therapeutic strategies for patients with refractory gastroesophageal reflux disease (GERD) – a, double blind, placebo- controlled trial. Gastroenterology. (2011) 140:S579. 10.1016/S0016-5085(11)62398-6 [DOI] [Google Scholar]
- 154.Rohof WO, Bennink RJ, de Ruigh AA, Hirsch DP, Zwinderman AH, Boeckxstaens GE. Effect of azithromycin on acid reflux, hiatus hernia and proximal acid pocket in the postprandial period. Gut. (2012) 61:1670–7. 10.1136/gutjnl-2011-300926 [DOI] [PubMed] [Google Scholar]
- 155.Krarup AL, Ny L, Gunnarsson J, Hvid-Jensen F, Zetterstrand S, Simrén M, et al. clinical trial: inhibition of theTRPV1 system in patients with non erosive gastroesophageal reflux disease and a partial response to PPI treatment is not associated with analgesia to esophageal experimental pain. Scand J Gastroenterol. (2013) 48:274–84. 10.3109/00365521.2012.758769 [DOI] [PubMed] [Google Scholar]
- 156.Riehl ME, Kinsinger S, Kahrillas PJ, Pandolfino JE, Keefer L. Role of a health psychologist in the management of functional esophageal complaints. Dis Esophagus. (2015) 28:428–36. 10.1111/dote.12219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Jannson C, Nordenstedt H, Wallander MA, Johansson S, Johnsen R, Hveem K, et al. Severe gastro-oesophageal reflux symptoms in relation to anxiety, depression and coping in a population based study. Aliment Pharmacol Ther. (2007) 26:683–91. 10.1111/j.1365-2036.2007.03411.x [DOI] [PubMed] [Google Scholar]
- 158.Becher A, El-Serag H. Systematic review: the association between symptomatic response to proton pump inhibitors and health-related quality of life in patients with gastro-oesophageal reflux disease. Aliment Pharmacol Ther. (2011) 34:618–27. 10.1111/j.1365-2036.2011.04774.x [DOI] [PubMed] [Google Scholar]
- 159.Weijenborg PW, de Schepper HS, Smout AJ, Bredenoord AJ. Effects of antidepressants in patients with functional esophageal disorders or gastroesophageal reflux disease: a systematic review. Clin Gastroenterol Hepatol. (2015) 13:251–9. 10.1016/j.cgh.2014.06.025 [DOI] [PubMed] [Google Scholar]
- 160.Ong AM, Chua LT, Khor CJ, Asokkumar R, Namasivayam V, Wang Y. Diaphragmatic breathing reduces belching and proton pump inhibitor refractory gastroesophageal reflux symtpmos. Clin Gastroenterol Hepatol. (2018) 16:407–16.e2. 10.1016/j.cgh.2017.10.038 [DOI] [PubMed] [Google Scholar]
- 161.Riehl ME, Pandolfino JE, Palsson OS, Keefer L. Feasibility and acceptability of esophageal- directed hypnotherapy for functional heartburn. Dis Esoph. (2016) 29:490–6. 10.1111/dote.12353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Chiarioni G, Withead WE. The role of biofeedback in the treatment of gastrointestinal disorders. Nat Clin Pract Gastroenterol Hepatol. (2008) 5:371–82. 10.1038/ncpgasthep1150 [DOI] [PubMed] [Google Scholar]
- 163.Patrick L. Gastroesophageal reflux disease (GERD): a review of conventional and alternative treatments. Altern Med Rev. (2011) 16:116–33. [PubMed] [Google Scholar]
- 164.Fuchs KH, Babic B, Breithaupt W, Dallemagne B, Fingerhut A, Furnee E, et al. European Association of Endoscopic Surgery (EAES). EAES recommendations for the management of gastroesophageal reflux disease. Surg Endosc. (2014) 28:1753–73. 10.1007/s00464-014-3431-z [DOI] [PubMed] [Google Scholar]
- 165.Grant AM, Boachie C, Cotton SC, Faria R, Bojke L, Epstein DM, et al. REFLUX trial group. Clinical and economic evaluation of laparoscopic surgery compared with medical management for gastro-oesophageal reflux disease: 5-year follow-up of multicentre randomised trial (the REFLUX trial). Health Technol Assess. (2013) 17:1–167. 10.3310/hta17220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Stefanidis D, Hope WW, Kohn GP, Reardon PR, Richardson WS, Fanelli RD. SAGES Guidelines Committee. Guidelines for surgical treatment of gastroesophageal reflux disease. Surg Endosc. (2010) 24:2647–69. 10.1007/s00464-010-1267-8 [DOI] [PubMed] [Google Scholar]
- 167.Weusten BLAM, Barret M, Bredenoord AJ, Familiari P, Gonzalez JM, van Hooft JE, et al. Endoscopic management of gastrointestinal motility disorders - part 1: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. (2020) 52:498–515. 10.1055/a-1160-5549 [DOI] [PubMed] [Google Scholar]
- 168.Weusten BLAM, Barret M, Bredenoord AJ, Familiari P, Gonzalez JM, van Hooft JE, et al. Endoscopic management of gastrointestinal motility disorders - part 2: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. (2020) 52:600–14. 10.1055/a-1171-3174 [DOI] [PubMed] [Google Scholar]
- 169.Dallemagne B, Weerts J, Markiewicz S, Dewandre JM, Wahlen C, Monami B, et al. Clinical results of laparoscopic fundoplication at ten years after surgery. Surg Endosc. (2006) 20:159–65. 10.1007/s00464-005-0174-x [DOI] [PubMed] [Google Scholar]
- 170.Jobe BA, Richter JE, Hoppo T, Peters JH, Bell R, Dengler WC, et al. Preoperative diagnostic workup before antireflux surgery: an evidence and experience-based consensus of the Esophageal Diagnostic Advisory Panel. J Am Coll Surg. (2013) 217:586–97. 10.1016/j.jamcollsurg.2013.05.023 [DOI] [PubMed] [Google Scholar]
- 171.Fernando HC. Endoscopic fundoplication: patient selection and technique. J Vis Surg. (2017) 3:121. 10.21037/jovs.2017.08.03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Moore M, Afaneh C, Benhuri D, Antonacci C, Abelson J, Zarnegar R. Gastroesophageal reflux disease: a review of surgical decision making. World J Gastrointest Surg. (2016) 8:77–83. 10.4240/wjgs.v8.i1.77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Patti MG, Allaix ME, Fisichella PM. Analysis of the causes of failed antireflux surgery and the principles of treatment: a review. JAMA Surg. (2015) 150:585–90. 10.1001/jamasurg.2014.3859 [DOI] [PubMed] [Google Scholar]
- 174.Maret-Ouda J, Wahlin K, El-Serag HB, Lagergren J. Association between laparoscopic antireflux surgery and recurrence of gastroesophageal reflux. JAMA. (2017) 318:939–46. 10.1001/jama.2017.10981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Jobe BA, Kahrilas PJ, Vernon AH, Sandone C, Gopal DV, Swanstrom LL, et al. Endoscopic appraisal of the gastroesophageal valve after antireflux surgery. Am J Gastroenterol. (2004) 99:233–43. 10.1111/j.1572-0241.2004.04042.x [DOI] [PubMed] [Google Scholar]
- 176.Håkanson BS, Lundell L, Bylund A, Thorell A. Comparison of laparoscopic 270° posterior partial fundoplication vs total fundoplication for the treatment of gastroesophageal reflux disease: a clinical trial. JAMA Surg. (2019) 154:479–86. 10.1001/jamasurg.2019.0047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Yadlapati R, Hungness ES, Pandolfino JE. Complications of Antireflux Surgery. Am J Gastroenterol. (2018) 113:1137–47. 10.1038/s41395-018-0115-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Richter JE. Let the patient beware: the evolving truth about laparoscopic antireflux surgery. Am J Med. (2003) 114:71–3. 10.1016/S0002-9343(02)01389-X [DOI] [PubMed] [Google Scholar]
- 179.Hinder RA, Libbey JS, Gorecki P, Bammer T. Antireflux surgery. Indications, preoperative evaluation, and outcome. Gastroenterol Clin North Am. (1999) 28:987–1005, viii. 10.1016/S0889-8553(05)70101-1 [DOI] [PubMed] [Google Scholar]
- 180.Horgan S, Pohl D, Bogetti D, Eubanks T, Pellegrini C. Failed antireflux surgery: what have we learned from reoperations? Arch Surg. (1999) 134:809–15. 10.1001/archsurg.134.8.809 [DOI] [PubMed] [Google Scholar]
- 181.Zhou T, Harnsberger C, Broderick R, Fuchs H, Talamini M, Jacobsen G, et al. Reoperation rates after laparoscopic fundoplication. Surg Endosc. (2015) 29:510–4. 10.1007/s00464-014-3660-1 [DOI] [PubMed] [Google Scholar]
- 182.Kandulski A, Weigt J, Caro C, Jechorek D, Wex T, Malfertheiner P. Esophageal intraluminal baseline impedance differentiates gastroesophageal reflux disease from functional heartburn. Clin Gastroenterol Hepatol. (2015) 13:1075–81. 10.1016/j.cgh.2014.11.033 [DOI] [PubMed] [Google Scholar]
- 183.Memon MA, Subramanya MS, Hossain MB, Yunus RM, Khan S, Memon B. Laparoscopic anterior versus posterior fundoplication for gastro-esophageal reflux disease: a meta-analysis and systematic review. World J Surg. (2015) 39:981–96. 10.1007/s00268-014-2889-0 [DOI] [PubMed] [Google Scholar]
- 184.Spechler SJ, Lee E, Ahnen D, Goyal RK, Hirano I, Ramirez F, et al. Long-term outcome of medical and surgical therapies for gastroesophageal reflux disease: follow-up of a controlled trial. JAMA. (2001) 285:2331–8. 10.1001/jama.285.18.2331 [DOI] [PubMed] [Google Scholar]
- 185.Arts J, Tack J, Galmiche JP. Endoscopic antireflux procedures. Gut. (2004) 53:1207–14. 10.1136/gut.2003.025460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Richter JE, Kumar A, Lipka S, Miladinovic B, Velanovich V. Efficacy of laparoscopic Nissen Fundoplication vs Transoral Incisionless Fundoplication or proton pump inhibitors in patients with gastroesophageal reflux disease: a systematic review and network meta-analysis. Gastroenterology. (2018) 154:1298–308.e7. 10.1053/j.gastro.2017.12.021 [DOI] [PubMed] [Google Scholar]
- 187.Håkansson B, Montgomery M, Cadiere GB, Rajan A, Bruley des Varannes S, Lerhun M, et al. Randomised clinical trial: transoral incisionless fundoplication vs. sham intervention to control chronic GERD. Aliment Pharmacol Ther. (2015) 42:1261–70. 10.1111/apt.13427 [DOI] [PubMed] [Google Scholar]
- 188.Chang KJ, Bell R. Transoral Incisionless Fundoplication. Gastrointest Endosc Clin N Am. (2020) 30:267–89. 10.1016/j.giec.2019.12.008 [DOI] [PubMed] [Google Scholar]
- 189.Testoni PA, Testoni S, Distefano G, Mazzoleni G, Fanti L, Passaretti S. Transoral incisionless fundoplication with EsophyX for gastroesophageal reflux disease: clinical efficacy is maintained up to 10 years. Endosc Int Open. (2019) 7:E647–54. 10.1055/a-0820-2297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Gerson L, Stouch B, Lobontiu A. Transoral incisionless fundoplication (TIF 2.0): a meta- analysis of three, controlled clinical trials. Chirurgia. (2018) 113:173–84. 10.21614/chirurgia.113.2.173 [DOI] [PubMed] [Google Scholar]
- 191.Trad KS, Barnes WE, Simoni G, Shughoury AB, Mavrelis PG, Raza M, et al. Transoral incisionless fundoplication effective in eliminating GERD symptoms in partial responders to proton pump inhibitor therapy at 6 months: the TEMPO clinical trial. Surg Innov. (2015) 22:26–40. 10.1177/1553350614526788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Hunter JG, Kahrilas PJ, Bell RC, Wilson EB, Trad KS, Dolan JP, et al. Efficacy of transoral fundoplication vs omeprazole for treatment of regurgitation in a controlled trial. Gastroenterology. (2015) 148:324–33.e5. 10.1053/j.gastro.2014.10.009 [DOI] [PubMed] [Google Scholar]
- 193.Trad KS, Barnes WE, Prevou ER, Simoni G, Steffen JA, Shughoury AB, et al. The TEMPO trial at 5 years: transoral fundoplication (TIF 2.0) is safe, durable, and cost-effective. Surg Innov. (2018) 25:149–57. 10.1177/1553350618755214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Huang X, Chen S, Zhao H, Zeng X, Lian J, Tseng Y, et al. Efficacy of transoral incisionless fundoplication (TIF) for the treatment of GERD: a systematic review with meta-analysis. Surg Endosc. (2017) 31:1032–44. 10.1007/s00464-016-5111-7 [DOI] [PubMed] [Google Scholar]
- 195.McCarty TR, Itidiare M, Njei B, Rustagi T. Efficacy of transoral incisionless fundoplication for refractory gastroesophageal reflux disease: a systematic review and meta-analysis. Endoscopy. (2018) 50:708–25. 10.1055/a-0576-6589 [DOI] [PubMed] [Google Scholar]
- 196.Kauer WK, Roy-Shapira A, Watson D, Sonnenschein M, Sonnenschein E, Unger J, et al. Preclinical trial of a modified gastroscope that performs a true anterior fundoplication for the endoluminal treatment of gastroesophageal reflux disease. Surg Endosc. (2009) 23:2728–31. 10.1007/s00464-009-0479-2 [DOI] [PubMed] [Google Scholar]
- 197.Zacherl J, Roy-Shapira A, Bonavina L, Bapaye A, Kiesslich R, Schoppmann SF, et al. Endoscopic anterior fundoplication with the Medigus Ultrasonic Surgical Endostapler (MUSE™) for gastroesophageal reflux disease: 6-month results from a multi-center prospective trial. Surg Endosc. (2015) 29:220–9. 10.1007/s00464-014-3731-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Kim HJ, Kwon CI, Kessler WR, Selzer DJ, McNulty G, Bapaye A, et al. Long-term follow-up results of endoscopic treatment of gastroesophageal reflux disease with the MUSE™ endoscopic stapling device. Surg Endosc. (2016) 30:3402–8. 10.1007/s00464-015-4622-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Sumi K, Inoue H, Kobayashi Y, Iwaya Y, Abad MRA, Fujiyoshi Y, et al. Endoscopic treatment of proton pump inhibitor-refractory gastroesophageal reflux disease with anti-reflux mucosectomy: experience of 109 cases. Dig Endosc. (2021) 33:347–54. 10.1111/den.13727 [DOI] [PubMed] [Google Scholar]
- 200.Benias PC, D'Souza L, Lan G, Gluckman C, Inamdar S, Trindade AJ, et al. Initial experience with a novel resection and plication (RAP) method for acid reflux: a pilot study. Endosc Int Open. (2018) 6:E443–9. 10.1055/s-0044-101453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Inoue H, Tanabe M, de Santiago ER, Abad MRA, Shimamura Y, Fujiyoshi Y, et al. Anti-reflux mucosal ablation (ARMA) as a new treatment for gastroesophageal reflux refractory to proton pump inhibitors: a pilot study. Endosc Int Open. (2020) 8:E133–8. 10.1055/a-1031-9436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Hernández Mondragón OV, Zamarripa Mottú RA, García Contreras LF, Gutiérrez Aguilar RA, Solórzano Pineda OM, Blanco Velasco G, et al. Clinical feasibility of a new antireflux ablation therapy on gastroesophageal reflux disease (with video). Gastrointest Endosc. (2020) 92:1190–201. 10.1016/j.gie.2020.04.046 [DOI] [PubMed] [Google Scholar]
- 203.Fortinsky KJ, Shimizu T, Chin MA, Kim J, Samarasena JB, Chang KJ. Tu1168 mucosal ablation and suturing at the esophagogastric junction (MASE): a novel procedure for the management of patients with gastroesophageal reflux disease. Gastrointest Endosc. (2018) 87:AB552 10.1016/j.gie.2018.04.2198 [DOI] [Google Scholar]
- 204.Weitzendorfer M, Spaun GO, Antoniou SA, Witzel K, Emmanuel K, Koch OO. Clinical feasibility of a new full-thickness endoscopic plication device (GERDx™) for patients with GERD: results of a prospective trial. Surg Endosc. (2018) 32:2541–9. 10.1007/s00464-018-6153-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Buckley FP 3rd, Bell RCW, Freeman K, Doggett S, Heidrick R. Favorable results from a prospective evaluation of 200 patients with large hiatal hernias undergoing LINX magnetic sphincter augmentation. Surg Endosc. (2018) 32:1762–8. 10.1007/s00464-017-5859-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Ganz RA. A modern magnetic implant for gastroesophageal reflux disease. Clin Gastroenterol Hepatol. (2017) 15:1326–37. 10.1016/j.cgh.2016.12.019 [DOI] [PubMed] [Google Scholar]
- 207.Rogers BD, Valdovinos LR, Crowell MD, Bell R, Vela MF, Gyawali CP. Number of reflux episodes on pH-impedance monitoring associates with improved symptom outcome and treatment satisfaction in gastro-oesophageal reflux disease (GERD) patients with regurgitation. Gut. (2021) 70:450–5. 10.1136/gutjnl-2020-321395 [DOI] [PubMed] [Google Scholar]
- 208.Smith CD, Ganz RA, Lipham JC, Bell RC, Rattner DW. Lower esophageal sphincter augmentation for gastroesophageal reflux disease: the safety of a modern implant. J Laparoendosc Adv Surg Tech A. (2017) 27:586–91. 10.1089/lap.2017.0025 [DOI] [PubMed] [Google Scholar]
- 209.Guidozzi N, Wiggins T, Ahmed AR, Hanna GB, Markar SR. Laparoscopic magnetic sphincter augmentation versus fundoplication for gastroesophageal reflux disease: systematic review and pooled analysis. Dis Esophagus. (2019) 32:doz031. 10.1093/dote/doz031 [DOI] [PubMed] [Google Scholar]
- 210.Skubleny D, Switzer NJ, Dang J, Gill RS, Shi X, de Gara C, et al. LINX® magnetic esophageal sphincter augmentation versus Nissen fundoplication for gastroesophageal reflux disease: a systematic review and meta-analysis. Surg Endosc. (2017) 31:3078–84. 10.1007/s00464-016-5370-3 [DOI] [PubMed] [Google Scholar]
- 211.Franciosa M, Mashimo H. Stretta radiofrequency treatment for GERD: a safe and effective modality. Am J Gastroenterol. (2013) 108:1654–5. 10.1038/ajg.2013.275 [DOI] [PubMed] [Google Scholar]
- 212.Auyang ED, Carter P, Rauth T, Fanelli RD, Committee SG. SAGES clinical spotlight review: endoluminal treatments for gastroesophageal reflux disease (GERD). Surg Endosc. (2013) 27:2658– 72. 10.1007/s00464-013-3010-8 [DOI] [PubMed] [Google Scholar]
- 213.Lipka S, Kumar A, Richter JE. No evidence for efficacy of radiofrequency ablation for treatment of gastroesophageal reflux disease: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. (2015) 13:1058–67.e1. 10.1016/j.cgh.2014.10.013 [DOI] [PubMed] [Google Scholar]
- 214.Fass R, Cahn F, Scotti DJ, Gregory DA. Systematic review and meta-analysis of controlled and prospective cohort efficacy studies of endoscopic radiofrequency for treatment of gastroesophageal reflux disease. Surg Endosc. (2017) 31:4865–82. 10.1007/s00464-017-5431-2 [DOI] [PubMed] [Google Scholar]
- 215.Zerbib F, Sacher-Huvelin S, Coron E, Coffin B, Melchior C, Ponchon T, et al. Randomised clinical trial: oesophageal radiofrequency energy delivery versus sham for PPI-refractory heartburn. Aliment Pharmacol Ther. (2020) 52:637–45. 10.1111/apt.15936 [DOI] [PubMed] [Google Scholar]
- 216.Das B, Reddy M, Khan OA. Is the Stretta procedure as effective as the best medical and surgical treatments for gastro-oesophageal reflux disease? A best evidence topic. Int J Surg. (2016) 30:19–24. 10.1016/j.ijsu.2016.03.062 [DOI] [PubMed] [Google Scholar]
- 217.Kappelle WF, Bredenoord AJ, Conchillo JM, Ruurda JP, Bouvy ND, van Berge Henegouwen MI, et al. Electrical stimulation therapy of the lower oesophageal sphincter for refractory gastro- oesophageal reflux disease - interim results of an international multicentre trial. Aliment Pharmacol Ther. (2015) 42:614–25. 10.1111/apt.13306 [DOI] [PubMed] [Google Scholar]
- 218.Talley NJ, Zand Irani M. Optimal management of severe symptomatic gastroesophageal reflux disease. J Intern Med. (2021) 289:162–78. 10.1111/joim.13148 [DOI] [PubMed] [Google Scholar]