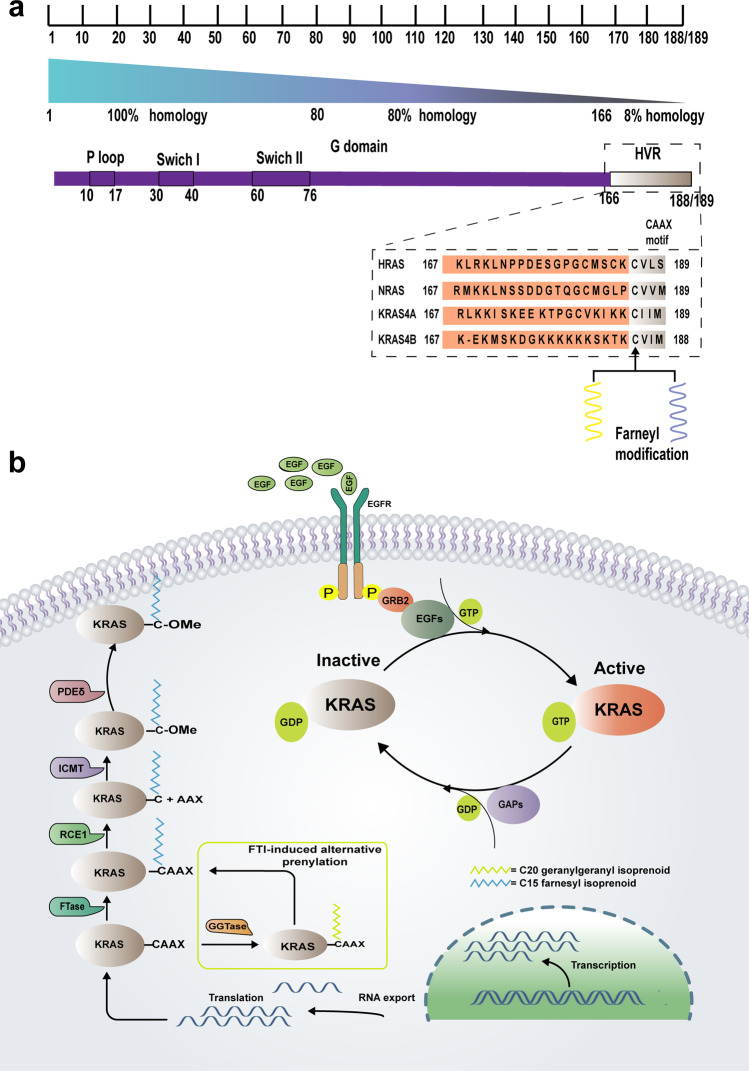
Fig. 1.
The structure and function of KRAS. a According to homology, KRAS, which consists of 188/189 amino acids can be divided into three parts. The first part consisting of the first 85 amino acid residues is a highly conserved region. The next 80 amino acid residues are defined as a second part where homology between any pair of human RAS genes is 85%. A third part is a highly variable region and homology is only 8%. KRAS forms two major domains: a catalytic domain called the G domain and a hypervariable region (HVR). The G domain consists of three regions: switch I, switch II and the P loop, which binds guanine nucleotides and activates signalling pathway by interacting with effectors. The HVR consists of a membrane-targeting domain containing the CAAX motif where C is a cysteine, A is any aliphatic amino acid and X is any amide acid, which acquires lipids by farnesyl or prenyl modification. b The normal function of KRAS depends on the membrane localisation of its post-transcriptional modification, which is mediated by a series of enzymes. KRAS functions as a guanosine diphosphate (GDP)/triphosphate (GTP) binary switch, which controls important signal transduction from activated membrane receptors to intracellular molecules. The binary switch is mainly determined by two kinds of regulatory proteins: guanine nucleotide exchange factors (GEFs) and GTPase-activating proteins (GAPs). FTase: farnesyltransferase; GGTase: geranyl geranyltransferase; RCE1: RAS-converting enzyme 1; ICMT: isoprenylcysteine carboxyl methyltransferase; PDEδ: phosphodiesterase δ