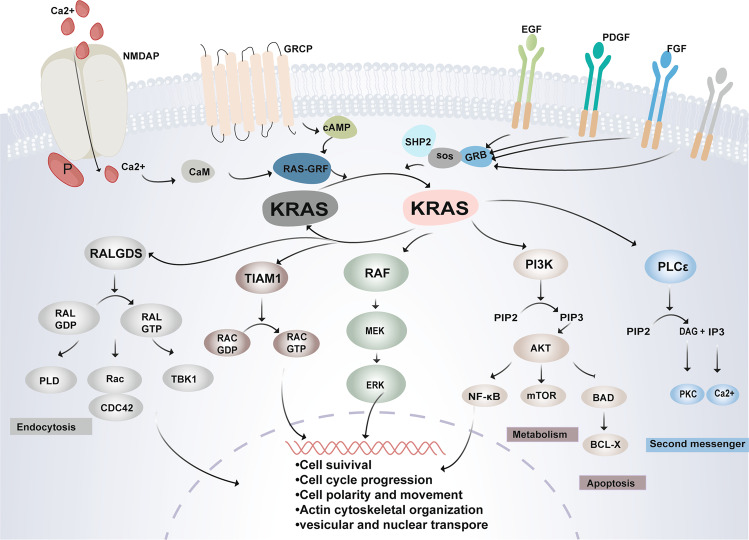
Fig. 2.
The regulation of KRAS activation and signal transduction. The canonical and well-known pattern of activating KRAS is dependent on correct membrane localisation and adjacent activation of membrane receptors. In the resting state, KRAS normally binds with GDP in an inactivated state. When the extracellular growth factors such as EGF transmit signals to receptors, the SOS, a kind of GEF, interacts with the KRAS-GDP complex leading to the release of GDP and the replacement of GTP. The tether of GTP and KRAS induces structural changes of switch I and switch II, thereby activating KRAS. In contrast, GAPs enhance intrinsic GTPase activity in KRAS to accelerate the reaction in which GTP is hydrolysed to GDP. The KRAS cycle between the activated and inactivated conformations functions as a finely regulated molecular switch that controls multiple signalling cascades, including the canonical RAF-MEK-ERK pathway, which controls proliferation; PI3K-AKT-mTOR pathway, which promotes cell survival; and other signalling pathways, which are required for KRAS-dependent tumour growth and endocytosis, and cytoskeletal organisation