Figure 4.
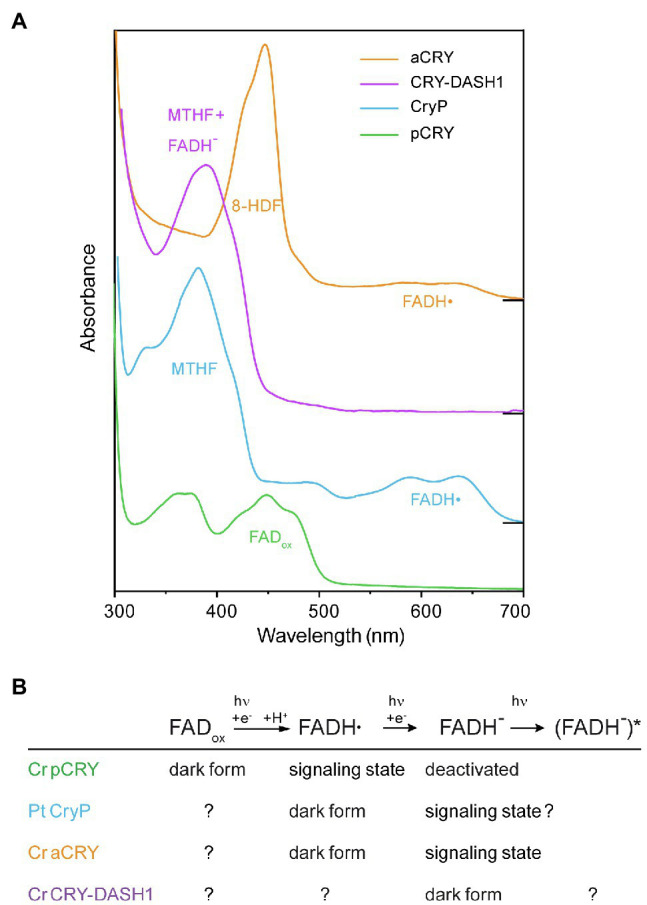
Absorption spectra of different cryptochromes from algae recorded in vitro directly after isolation and the postulated roles of the redox states of FAD in these cryptochromes. (A) The absorption spectra of cryptochromes are characterized by the different oxidation states of the FAD chromophore and the binding of antenna molecules. The plant cryptochrome Cr pCRY binds FADox and acts as blue-light receptor. Plant-like cryptochrome Pt CryP and animal-like cryptochrome Cr aCRY both absorb almost in the full visible spectral region because of the absorption of FADH•. However, Pt CryP binds MTHF as antenna, whereas Cr aCRY binds 8-HDF leading to different absorption maxima in the UV-A and blue region, respectively. The UV-A-receptor Cr CRY-DASH1 absorbs mainly at around 380nm dominated by the contribution of MTHF, whereas its chromophore FADH− absorbs only weakly. For simplicity, the absorption of FADox, FADH• and FADH− is indicated at the maximum with highest wavelength, but all FAD species also contribute to the absorption at lower wavelengths. Spectra were displaced vertically for better visibility. (B) The redox states of FAD play different roles in the algal cryptochromes, as postulated on the basis of the absorption spectra and functional studies of representative members. The dark form indicates the redox state present in vivo in the dark. Light absorption induces a reduction of the FAD. The signaling state is associated with conformational changes and/or changes in oligomerization state of the receptor that drive signal transduction.
