Figure 3. New regulatory layers unveiled by the VAV1 mutations.
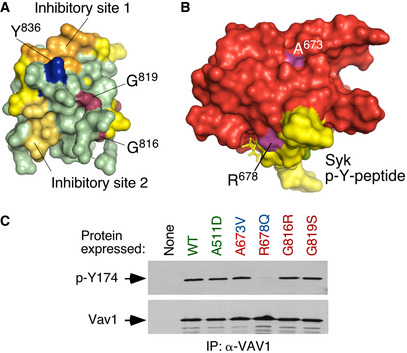
- 3D structure of the Vav1 CSH3 domain. The previously described intramolecular inhibitory sites 1 and 2 of the Vav1 CSH3 domain are depicted in orange and yellow, respectively (Barreira et al, 2014). The regulatory Y836 phosphosite is highlighted in blue (Barreira et al, 2014). The residues present in the new inhibitory interface of the CSH3 are shown in raspberry color.
- 3D structure of the VAV1 SH2 (red) bound to a Syk tyrosine‐phosphorylated peptide (yellow). The mutated residues are shown in purple. p‐Y, tyrosine phosphorylated.
- Levels of phosphorylation of the Y174 phosphosite of the indicated Vav1 mutant proteins immunoprecipitated from Jurkat cells (upper panel). The total amount of Vav1 immunoprecipitated in each sample is shown in the bottom panel (n = 3 independent experiments).
Source data are available online for this figure.
