Abstract
Self-grooming is an innate, cephalo-caudal progression of body cleaning behaviors that are found in normal rodents but exhibit repetitive and stereotyped patterns in several mouse models, such as autism spectrum disorders (ASDs). It is also recognized as a marker of stress and anxiety. Mice with Shank3B gene knockout (KO) exhibit typical ASD-like behavioral abnormalities, including stereotyped self-grooming and increased levels of anxiety. However, the exact relationship between anxiety and stereotyped self-grooming in certain types of animal models is not clear. We selected three animal models with high anxiety to compare their self-grooming parameters. First, we confirmed that Shank3B KO mice (ASD model), acute restraint stress mouse model (stress model), and chronic inflammatory pain mouse model (pain model) all showed increased anxiety levels in the open field test (OFT) and elevated plus maze (EPM). We found that only the ASD model and the stress model produced increased total grooming duration. The pain model only exhibited an increasing trend of mean self-grooming duration. We used the grooming analysis algorithm to examine the self-grooming microstructure and assess the cephalo-caudal progression of grooming behavior. The results showed distinct self-grooming microstructures in these three models. The anxiolytic drug diazepam relieved the anxiety level and the total time of grooming in the ASD and stress models. The grooming microstructure was not restored in Shank3B KO mice but was partially relieved in the stress model, which suggested that anxiety aggravated stereotyped self-grooming duration but not the grooming microstructure in the ASD mouse model. Our results indicated that stereotyped behavior and anxiety may be shared by separate, but interacting, neural circuits in distinct disease models, which may be useful to understand the mechanisms and develop potential treatments for stereotyped behaviors and anxiety.
Keywords: Stereotyped self-grooming, Anxiety, Shank3, Restraint, Inflammatory pain
1. Introduction
Stereotyped behaviors are defined as repetitive, coordinated, seemingly purposeless, rhythmic behaviors (Lai et al., 2014; Rapp and Vollmer, 2005). These behaviors can be found in normal people but are also typical symptoms in several psychiatric disorders, such as autism spectrum disorders (ASDs) (Uljarević et al., 2021) and obsessive-compulsive disorders (OCD) (Pertusa et al., 2008). They may cause the patients to lose educational opportunities and be excluded from social interactions (Taylor et al., 2005). To understand the neural mechanisms of the stereotyped behaviors, the proper and correlated phenotype in animal models is necessary. Self-grooming is a common stereotyped behavior that is observed in many animal species, especially rodents (Arakawa, 2021; Askari-Zahabi et al., 2021). It follows an innate cephalo-caudal progression, beginning with paw licking, then nose/face/head washing, body grooming, leg licking, and finally tail/genitals grooming, which display subtle hierarchical motor control (Kalueff et al., 2007). And the quantity and progression of self-grooming were found to be changed in several rodent models of neuropsychiatric disorders (Kalueff et al., 2016). For example, stereotyped self-grooming is known as one of the core symptoms of several ASDs mouse models (Meyza et al., 2013; Peca et al., 2011). And anxiety and stress were also found to be accompanied by increased self-grooming behaviors in rodents (Estanislau et al., 2013).
One particularly interesting thing is that anxiety is also common emotional comorbidity in many diseases, such as ASDs, chronic pain, and stress states (Guo et al., 2018; Mosaffa et al., 2021; van Steensel et al., 2011). It raises a question: what's the relationship between anxiety and stereotyped behaviors. In clinical, some pieces of evidence suggest that anxiety and stereotyped behaviors are separate and distinct categories (Gotham et al., 2013; Renno and Wood, 2013). However, several studies suggested that children with high anxiety had more stereotyped behaviors and vice versa (Baribeau et al., 2020, 2021; Rodgers et al., 2012). In animal models, self-grooming has long been recognized as a marker of stress (Moody et al., 1988; Mu et al., 2020), and stress and anxiety were intertwined (Daviu et al., 2019). Recent work found that mice with Shank3 gene knockout (KO), an ASDs mouse model, exhibited stereotyped self-grooming and increased anxiety (Peca et al., 2011). These associations suggest that stereotyped self-grooming may be due to its emotional comorbidity-anxiety. However, the reciprocal interaction between stereotyped self-grooming and anxiety is not known.
To better understand the relationship between excessive self-grooming and anxiety, we selected three mouse models with increasing anxiety-like behavior: Shank3B KO ASDs mouse model (Peca et al., 2011), acute restraint stress mouse model (Mosaffa et al., 2021), and chronic inflammatory pain mouse model induced by complete Freund's adjuvant (CFA) (Guo et al., 2018) to achieve our goals. The aims of the present study were: (a) to compare the self-grooming changes in different anxiety mouse models by analyzing classical grooming parameters and the cephalo-caudal grooming microstructure (Kalueff et al., 2007; Kalueff and Tuohimaa, 2005); (b) to understand the roles of anxiety in self-grooming by using an anxiolytic medicine diazepam; (c) to compare the activated brain regions in the progress of self-grooming by using c-Fos labeling strategy.
2. Material and methods
2.1. Animals
Adult male C57BL/6 mice aged 6–8 weeks were obtained from the Laboratory Animal Center of the Fourth Military Medical University (Xi'an, China). Shank3B KO mice were obtained from Prof. Guoping Feng (Peca et al., 2011). The mice were housed at a constant temperature (22–25 °C) in an environment on a 12-h light/dark cycle (lights on at 08:00 and off at 20:00). Food and water were available ad libitum. The Institutional Animal Care and Use Committee (IACUC) of the FMMU approved all experimental procedures, which were performed in accordance with the “Principles of Medical Laboratory Animal Care” issued by the National Ministry of Health in China. All efforts were made to minimize the number of animals used and reduce animal suffering.
2.2. Animal models
2.2.1. Restraint stress-induced anxiety model (stress model)
Anxiety was produced by restraining mice in tubes (Garcia-Keller et al., 2021). Briefly, mice were placed into a modified 50-mL Falcon tube with breather holes with only the tail and tip of the nose free for 30 min. At the end of restraint, animals were immediately transferred to a grooming chamber or open field test equipment for behavioral testing. Control animals were left in their home cages for the same amount of time. Each mouse was restrained only once in the entire testing process.
2.2.2. CFA-induced chronic inflammatory pain model (pain model)
The CFA-induced chronic inflammatory pain model was established as previously described (Guo et al., 2018). Mice were anesthetized with isoflurane. CFA (10 μL) was injected into the plantar left hindpaw of mice, and the needle was left in place for 2 min after injection. Control animals received injections with saline (0.9% sodium chloride). Behavioral tests were performed at least 24 h later.
2.3. Behavioral procedures
All behavioral tests were performed in a sound-attenuated room with low-intensity light of approximately 30 lux. Observers were unaware of the treatment applied. Animals were acclimated to the holding room for at least 1 h before behavioral testing.
2.3.1. Open field test (OFT)
An OFT was performed to detect anxiety and locomotor activity, as previously described (Kraeuter et al., 2019b). Animals were individually placed in the central area of box (50 × 50 × 50 cm) and recorded for 10 min by a camera located above the box. The apparatus floor was divided into 16 equal parts, and the 4 middle parts were merged as the central area. The time and entries spent in the center and total distance traveled were measured using an automated analysis system (SMART 3.0, Panlab S.L.U., Spain).
2.3.2. Elevated plus-maze (EPM)
The EPM is used to assess the anxiety responses of mice (Kraeuter et al., 2019a). The plus maze apparatus was placed 90 cm above the floor and consisted of two open arms (25 × 8 × 12 cm), two closed arms (25 × 8 × 12 cm), and a junction platform (8 × 8 cm). Mice were gently placed at the center of the platform facing the open arms. The activity and travel trace of mice were measured for 5 min by a camera located above the apparatus. In EPM, experimental animals with low anxiety will spend more time in the open arms than control animals (Guo et al., 2018). It is worth noting that parameters of EPM had different meaning: the percentage of time spend in open arms and percentage of open arms entries in total entries (open arms entries + close arms entries) were taken as anxiety factors, but entries in close arms were used for motor activity (Cruz et al., 1994). We analyze all these three parameters. An entry in the open arm is defined as all four paws are in the area.
2.3.3. Grooming behavior analysis
The self-grooming behavior was videotaped for 30 min from a side view using an FPV camera (IMA238, SONY, Japan) in a small transparent plastic box (20 × 20 × 25 cm). Four mice were simultaneously recorded in four separate uniform boxes with similar illumination (30 lux) and background noise. The testing boxes were cleaned with 75% ethanol after the recording was finished. A manual score was determined by observers who were blind to the animal group using BORIS (version 7.9) (Friard and Gamba, 2016). For classical analysis of grooming activity, the following parameters were evaluated: the total time spent grooming, the number of grooming bouts, and mean grooming duration in one bout. A new grooming bout started when the last grooming behavior terminated for more than 6 s (Kalueff et al., 2007). The grooming patterns were analyzed based on the previous report by Kalueff and Tuohimaa (2004). The modified grooming microstructure was analyzed using the following scaling system: (stage 0) no grooming, (stage 1) paw licking, (stage 2) nose/face/head wash, (stage 3) body grooming, (stage 4) leg licking, and (stage 5) tail/genitals grooming (Kalueff et al., 2007). In briefly, paw licking is that licking front paws with the mouth, nose/face/head wash is that front paws stroke along the snout/head/behind ears, body grooming is body fur licking, leg licking is that licking hind paws with the mouth and tail/genitals grooming is licking of the genital area and tail. Interestingly, in a complete grooming process, mice might stop grooming for a brief duration then continue grooming, this period is usually shorter than 6 s, called no grooming stage, it goes into a new bout once terminated duration is more than 6s.
2.3.4. CFA-induced inflammatory pain behaviors
Mechanical sensitivity was assessed by measuring paw withdrawal thresholds (PWTs) with a series of von Frey filaments using the “up-down” method (quantitative assessment of tactile allodynia in the mouse paw) (Guo et al., 2018). After habituation to individual Plexiglas compartments for 1 h, the plantar surface of the mouse left hindpaw was stimulated by a series of von Frey filaments ranging from 0.07 g to 1.4 g. A positive response was considered a brisk withdrawal or paw flinching. PWTs were repeated five times in each mouse, and a 50% withdrawal threshold was analyzed.
The thermonociceptive threshold was measured using an instrument for plantar analgesia. The mice were put in individual plastic boxes for 30 min. The paw withdrawal latencies (PWLs) in response to a radiant heat source were collected. The PWLs were tested within 20 s to prevent tissue damage. The test was repeated 3 times with a 5-min interval in each mouse.
2.4. c-Fos experiments
The transcription factor c-Fos is widely used as a functional marker of activated neurons (Jaworski et al., 2018). We used c-Fos to identify the brain regions that were activated during grooming behavior. All mice used for c-Fos staining were acclimated to the holding room for at least 1 h before behavioral testing. And to decline the influence of experimental operation, we recorded and operated the experimental group and control group simultaneously. For Shank3B KO mice and its control group, after the acclimation, we immediately recorded grooming behavior for 30 min, and the mice were kept in their recording chamber until they were sacrificed 1.5 h after the grooming behavior. For the restraint stress-induced anxiety model, mice were first restrained for 30 min and then recorded grooming behavior for 30 min. Similar to Shank3B KO mice, restraint mice were anesthetized 1.5 h later with 1% sodium pentobarbital and intracardially perfused with 4% paraformaldehyde in phosphate buffered saline (PBS, pH 7.4). Brains were post-fixed for 2 h and cryoprotected in 30% sucrose for 3 days. 50 μm coronal sections were obtained from a cryostat and processed for c-Fos immunohistochemistry. In order to explore the brain regions that activated during self-grooming behavior as much as possible, we chose the brain slices with interval of 200 μm, and the majority of cerebral cortex, thalamus and amygdala were included. The slices were then washed with PBS for three times and incubated in a blocking solution for 2 h at room temperature and incubated overnight (4 °C) with primary antibody rabbit anti-c-Fos (1:1500, Cell Signaling Technology), and then the secondary antibody donkey anti-rabbit conjugated with Alexa Fluor 488 (1:500, Invitrogen) was used at room temperature for 4 h after washed with PBS. Images were obtained by microscope objective (FV3000, Olympus, Japan) and subsequent processing was in Imaris software (version 7.7, Bitplane, Switzerland) with its surface and spot motifs.
2.5. Drugs and injection
Diazepam (Tocris, USA) was dissolved in 0.9 % saline. The drugs were given intraperitoneally (i.p.) 30 min before the behavioral tests in a concentration of 1 mg/kg (Kalinina et al., 2016). All solutions were freshly prepared prior to injection and protected from light. CFA was purchased from Sigma (1 mg/mL Mycobacterium Tuberculosis; Sigma, UK).
2.6. Statistical analysis
All results were analyzed using SPSS (version 21.0, IBM, United States) and were graphed using Prism (version 6.0, GraphPad Software Inc., United States). The data are expressed as the means ± SEM. Normality (Shapiro–Wilk test) and homogeneity of variance (Levene's test) were tested first for statistical analyses. Student's t-test was used in EPM, OFT and grooming behavior data only when the date met both the normality test and the homogeneity of variance test. Datasets that did not meet the normality test were analyzed with a nonparametric test (Mann-Whitney U test). Datasets that met the normality test but not the homogeneity of variance test were analyzed with a separate variance estimation t-test. Mechanical hypersensitivity and thermal hypersensitivity were evaluated using Friedman's M test. p < 0.05 was considered statistically significant. All statistical data are presented in the Supplementary Table 1.
3. Results
3.1. The Shank3B KO autistic mouse model (ASD model), stress model, and pain model induced increased anxiety but distinct self-grooming behaviors
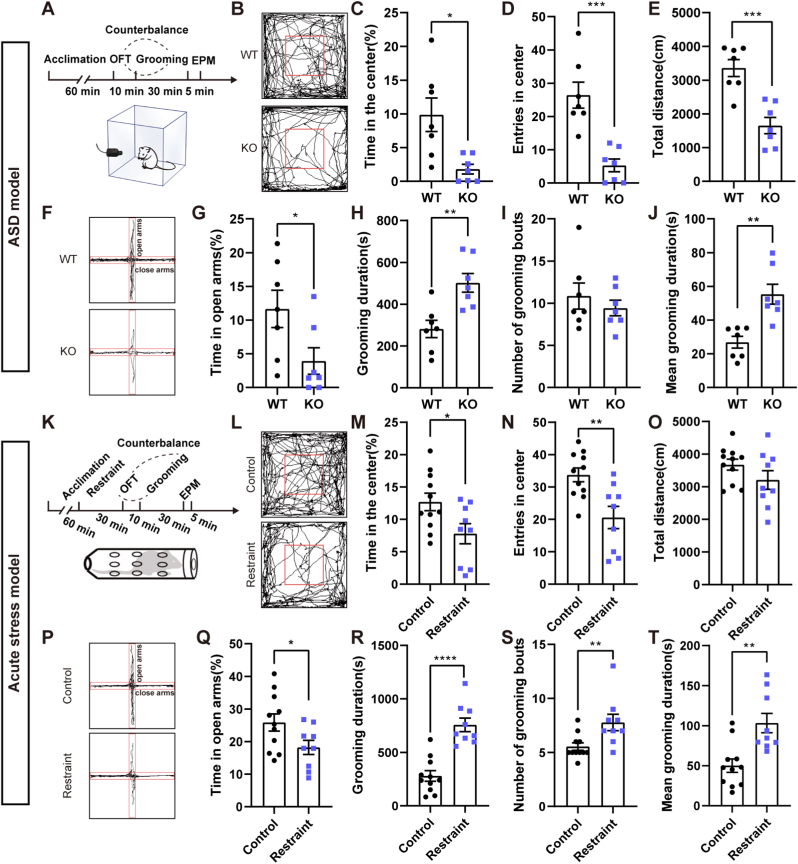
We tested anxiety-like behavior and self-grooming in Shank3B KO mice (Fig. 1A). The OFT results showed a decreasing percentage of time in the center area (t = 3.108, p < 0.05; n = 7 mice per group) and entries in the center area (t = 4.847, p < 0.001) in KO mice compared to wild-type (WT) littermates. The total distance of KO mice was also reduced (t = 4.876, p < 0.001) (Fig. 1B–E). The EPM showed that the percentage of time KO mice spent in the open arms (Z = −2.239, p < 0.05) (Fig. 1F–G) and the percentage of entries in open arms (t = 6.341, p < 0.0001) (Supplementary Fig. 1A) were also reduced. On the contrary, entries in close arms (t = −4.347, p < 0.001) (Supplementary Fig. 1B) were increased. These results suggested that Shank3B KO mice showed increased anxiety-like behavior and reduced locomotor activity. We analyzed the self-grooming behavior of KO mice and WT littermates. We found that the total grooming time of the KO mice at 30 min was significantly increased compared to the WT mice (t = −3.625, p < 0.01) (Fig. 1H). The number of grooming were similar between these two groups (t = 0.792, p = 0.444) (Fig. 1I). The mean grooming duration was increased in the KO groups (t = −4.154, p < 0.01) (Fig. 1J). These results confirmed that Shank3B KO mice had a high level of anxiety and stereotyped self-grooming behavior.
Fig. 1.
The Shank3B KO autistic mouse model (ASD model) and stress model showed increased anxiety but distinct self-grooming behaviors.
A, Schematic diagram of the behavioral testing protocol in the ASD model. B-E, Trajectory trials and OFT results in the ASD model. C-D, The percentage of time (Two-tailed unpaired separate variance estimation t-test) and entries in the center area of KO mice was reduced compared to wild-type (WT) mice (unpaired t-test, n = 7 mice per group). E, The total distance traveled by KO mice in the OFT was also reduced. F-G, Trajectory trials and EPM results in the ASD model. G, The percentage of time KO mice spent in the open arms of the EPM was reduced (Mann-Whitney U test, n = 7 mice per group). H, The total grooming time of KO mice at 30 min was significantly increased. I, The numbers of grooming bouts were similar between these two groups. J, The mean grooming duration showed an increase in the KO groups (unpaired t-test, n = 7 mice per group). K, Schematic diagram of the behavioral testing protocol in the stress model. L-O, Trajectory trials and results of OFT in the ASD model. M-N, The percentage of time and entries in the center area of restraint mice was reduced (unpaired t-test, control group n = 11 mice, restraint group n = 9 mice) O, The total distance traveled by restraint mice in the OFT was not changed. P-Q, Trajectory trials and results of EPM in stress model. Q, The percentage of time restraint mice spent in the open arms of the EPM was reduced. R-T, The total grooming time (unpaired t-test), number of grooming bouts (Mann-Whitney U test), and the mean grooming duration (Mann-Whitney U test) were all significantly enhanced in restraint mice. Data are shown as the means ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001 ****p < 0.0001.
We introduced two types of mouse models with severe anxiety-like behavior (acute restraint stress mouse model and chronic inflammatory pain mouse model) to further investigate the possible relationship between anxiety and stereotyped self-grooming behavior. The anxiety-like behavior and self-grooming in the acute restraint stress mouse model were tested (Fig. 1K). The restraint mice showed a reduction in the percentage of time (t = 2.674, p < 0.05; control group n = 11 mice, restraint group n = 9 mice) and entries into the center area (t = 3.399, p < 0.01) (Fig. 1L–N) without significant changes in the total distance traveled in the OFT (t = 1.195, p = 0.104) (Fig. 1O). The percentage of time spent in the open arms of restraint mice was also reduced in the EPM (t = 2.144, p < 0.05) (Fig. 1P–Q), but there is no influence of percentage of entries in open arms (t = 1.143, p = 0.268) and entries in close arms (t = −1.757, p = 0.096) (Supplementary Figs. 1C–D). The total grooming time (t = 6.804, p < 0.0001), number of grooming bouts (Z = −2.798, p < 0.01), and mean grooming duration (Z = −3.250, p < 0.01) were all significantly enhanced in the restraint mice (Fig. 1R–T). We checked the anxiety-like behavior and self-grooming in the chronic CFA-induced inflammatory pain model (Supplementary Fig. 1E). The CFA group showed significant mechanical (von Frey test) (χ2 = 21.552, p < 0.0001) and thermal (Hargreave's test) hyperalgesia (χ2 = 12.600, p < 0.001), which continued to the 12th day compared to the control group (Supplementary Figs. 1F–G). We also found that the CFA treatment increased the anxiety-like behavior by decreasing the percentage of time (t = 4.061, p < 0.01; -control group n = 8 mice, CFA group n = 7 mice) and entries in the center area (Z = −2.555, p < 0.05) but without significant changes in the total distance traveled in the OFT (t = 0.793, p = 0.449) (Supplementary Figs. 1H–K). The percentage of time spent in the open arms of the EPM was also reduced (Z = −1.969, p < 0.05) (Supplementary Fig. 1L–M), but percentage of entries in open arms (t = 0.538, p = 0.603) and entries in close arms (t = 0.308, p = 0.763) are similar (Supplementary Fig. 1N–O). However, the grooming duration of the CFA mice was not changed (t = −1.249, p = 0.235), and there was only an increasing trend of mean grooming duration (t = −2.332, p = 0.056), but it did not reach the significant difference caused by the opposite changes from the number of grooming bouts (Z = −2.494, p < 0.05) (Supplementary Fig. 1P–R).
Taken together, these results demonstrated that all three mouse models induced increased anxiety-like behavior. However, self-grooming presented different changes in these mouse models. Specifically, grooming was increased in the ASD model and the stress model, but it also showed some variance between these two models. However, the pain model did not induce obvious changes in stereotyped self-grooming. Thus, we will focus our attention more on ASD model and the stress model.
3.2. The ASD model exhibited a distinct microstructure in the stereotyped self-grooming phenotype compared to the stress model
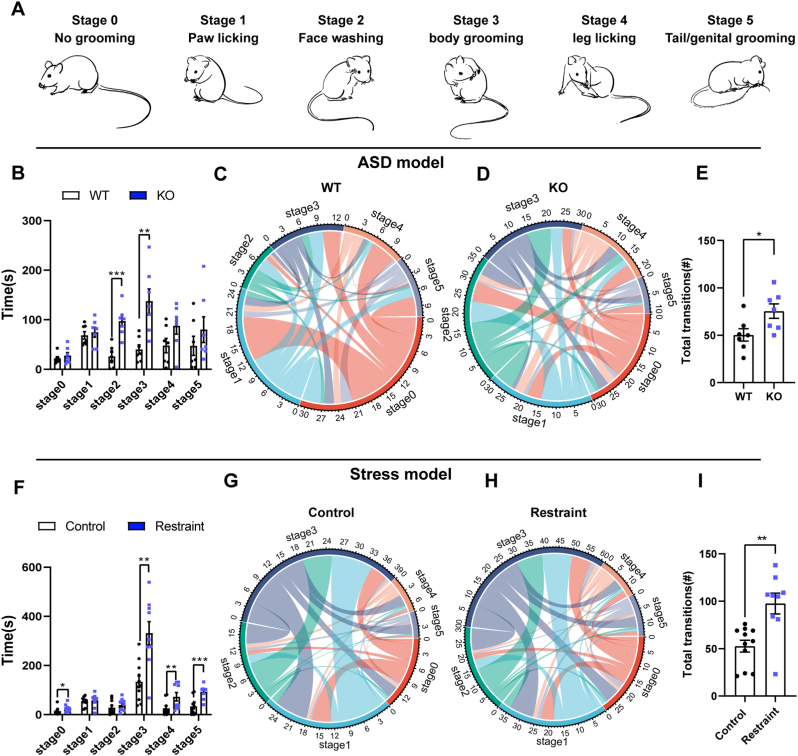
We sought more detail to explain the differences in stereotyped self-grooming behavior between the ASD model and stress model. Self-grooming behavior is an innate stereotyped cephalo-caudal microstructure in rodents that begins with paw licking and progresses to nose/face/head washing, body grooming, leg licking, and finally tail/genitals grooming. We established the following groups for these microstructures: stage 0 was no grooming; stage 1 was paw licking; stage 2 was face washing; stage 3 was body grooming; stage 4 was leg licking; and stage 5 was tail/genital grooming (Fig. 2A). We first compared the microstructure of self-grooming between Shank3B KO mice and their WT littermates. We found that the KO mice spent more time in stage 2 (t = −5.084, p < 0.001; n = 7 mice per group) and stage 3 grooming (t = −3.702, p < 0.01) than the WT mice (Fig. 2B). Previous studies suggested that anxiety-related grooming showed a chaotic pattern and defined the cephalo-caudal sequence (Kalueff and Tuohimaa, 2004). We also analyzed the switching between different stages of the self-grooming microstructure in WT and KO mice. A sequence switch was the major type of shifting in the microstructure of WT mice, such as a switch from stage 0 to stage 1, stage 3, stage 4, and stage 5, stage 1 to stage 2, stage 3. There were some relatively large reverse switches, including stage 1 to stage 0 and stage 5 to stage 0 (Fig. 2C). The increased switches in the KO group focused on stage 1, stage 2, stage 3, and stage 4, including sequence order and reverse order. For example, the switch from stage 0 to stage 2, stage 1 to stage 2, stage 2 to stage 3, stage 3 to stage 4, stage 2 to stage 1, stage 2 to stage 3, and stage 3 to stage 2 (Fig. 2D). In general, total transitions of these stages were more in KO group than WT group (t = −2.494, p < 0.05) (Fig. 2E). These results suggested that the grooming microstructure of Shank3B KO mice was characterized by an increase in stages 2 and 3, and a stage 2, 3-centered chaotic switching pattern.
Fig. 2.
The ASD model exhibited distinct microstructure of repetitive self-grooming phenotype compared to the stress model.
A, In rodents, self-grooming behavior is an innate stereotyped cephalo-caudal microstructure of 5 stages. Stage 0 is no grooming. Stage 1 is paw licking. Stage 2 is face washing. Stage 3 is body grooming. Stage 4 is leg licking. And stage 5 is tail/genital grooming. B, KO mice spent more time in stage 2 and stage 3 grooming than WT mice (unpaired t-test, n = 7 mice per group). C-D, In the KO group, there were increased switches focusing on stage 1, stage 2, stage 3 and stage 4, including sequence order and reverse order. E, Total transitions of switch were increased in the KO group (unpaired t-test, n = 7 mice per group). F, Restraint mice spent increased time in stage 3, stage 4 and stage 5 grooming. G-H, The reverse order distribution of the microstructure in restraint mice was increased compared to control mice. I, The restraint mice exhibited higher switch frequency (Mann-Whitney U test, control group n = 11 mice, restraint group n = 9 mice). Data are shown as the means ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001.
We checked the distribution and the switch pattern of the self-grooming microstructure in the stress model. The results showed that stage 3 (t = −3.958, p < 0.01; control group n = 11 mice, restraint group n = 9 mice), stage 4 (Z = −2.622, p < 0.01), and stage 5 (t = −4.338, p < 0.001) were the main stages of the grooming microstructure that were increased in the restraint mouse model (Fig. 2F). For the switch pattern of the grooming microstructure, stage 3 was the core in the restraint mice and the control mice, with switching from other stages to stage 3, including sequence and reverse order, as a major pattern. However, the restraint mice exhibited a higher number of switches (Z = −3.041, p < 0.01) (Fig. 2G–I). We explored the distribution and switch pattern of the self-grooming microstructure in the chronic CFA pain model. We found that the distributions of the self-grooming microstructure were similar between the CFA group and the control group (Supplementary Fig. 2A). But the switch and reverse order distribution of the microstructure in CFA group was higher than control group (t = −2.227, p < 0.05) (Supplementary Figs. 2B–D).
Taken together, these results indicated that the Shank3B KO autistic mouse model and stress model had diverse distributions and switch patterns in the microstructure of stereotyped self-grooming.
3.3. Stereotyped self-grooming in the ASD model and stress model was reduced partially and differently when anxiety-like behaviors were relieved by diazepam
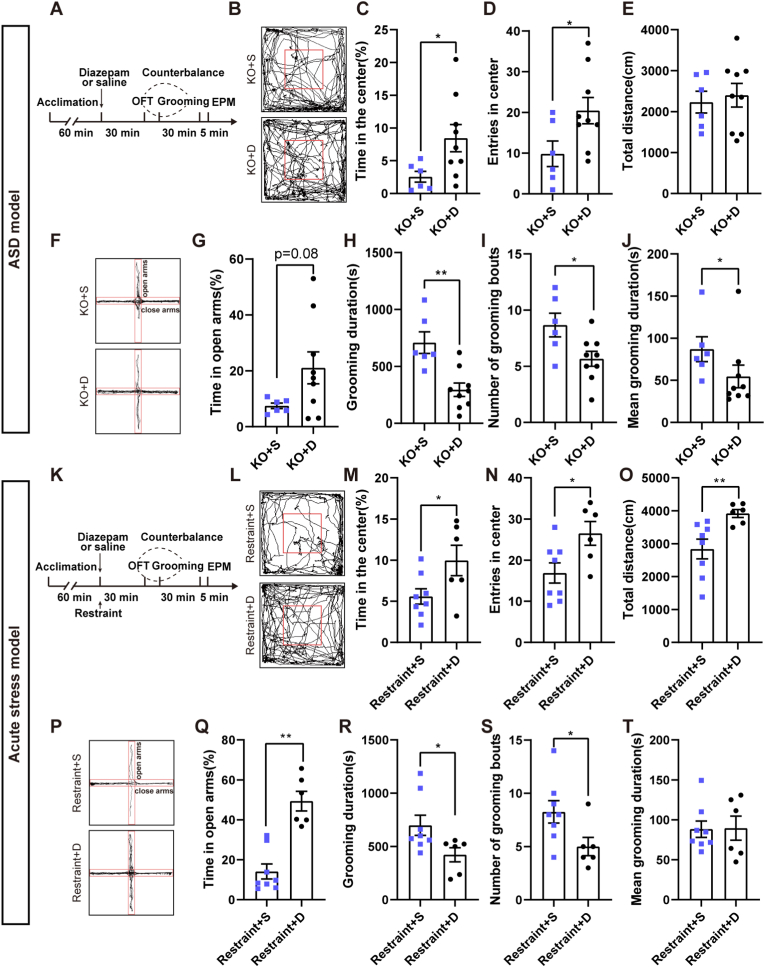
We examined whether relieving anxiety-like behavior alleviated the increased grooming behaviors. Diazepam is a benzodiazepine that has an anxiolytic effect in clinical patients and anxiety mouse models (Choleris et al., 2001). We examined the effect of diazepam on anxiety-like behavior and stereotyped self-grooming in three mouse models. We first tested the effect of diazepam on anxiety and stereotyped self-grooming in the ASD model (Fig. 3A). The results showed that diazepam treatment significantly increased the percentage of time spent in the center area (t = 2.568, p < 0.05; KO + S group n = 6 mice, KO + D group n = 9 mice) and entries into the center area (t = 2.249, p < 0.05) in KO mice without affecting the total distance traveled (t = 0.920, p = 0.379) (Fig. 3B–E). There was also a tendency to increase the percentage of time spent in the open arms in EPM after diazepam treatment (t = 2.205, p = 0.076) (Fig. 3F–G) and a significant increase of percentage of entries in open arms (t = −3.277, p < 0.01) (Supplementary Fig. 3A) as well as a significant decrease of entries in close arms (Z = −2.725, p < 0.01) (Supplementary Fig. 3B). This result suggested that diazepam relieved the anxiety-like behavior of Shank3B KO mice. We compared the effect of diazepam on stereotyped self-grooming in KO mice. We found that diazepam treatment reduced stereotyped self-grooming in KO mice (Fig. 3H–J). We tested the effects of diazepam on anxiety and self-grooming in the stress model (Fig. 3K). We found that the percentage of time spent in the center area (t = −2.286, p < 0.05; Restraint + S group n = 8 mice, Restraint + D group n = 6 mice), the number of entries into the center area (t = −2.537, p < 0.05), and the total distance traveled by the mice (t = −3.358, p < 0.01) treated with diazepam were all increased in the OFT compared to the saline group (Fig. 3L–O). The percentage of time in the open arms (Z = −3.098, p < 0.01) (Fig. 3P–Q) and percentage of entries in open arms (t = −7.335, p < 0.0001) (Supplementary Fig. 3C) of the EPM was increased in the mice treated with diazepam compared to the saline group. On the contrary, entries in close arms (t = 3.186, p < 0.01) (Supplementary Fig. 3D) were decreased. The restraint mice also showed reduced total grooming time in 30 min (Z = −2.195, p < 0.05) (Fig. 3R), which was due to a decreasing number of grooming bouts (t = 2.281, p < 0.05) but not mean grooming duration (t = 0.073, p = 0.943) (Fig. 3S-T). We also explored the effect of diazepam in the CFA pain model, although chronic pain did not increase grooming behavior (Supplementary Fig. 3E). The results demonstrated that diazepam treatment increased the percentage of time spent in the center area (t = −2.202, p < 0.05) but not the number of entries into the center area (t = −1.279, p = 0.222) or the total distance traveled compared with saline-injected CFA mice (t = −0.112, p = 0.913) (Supplementary Figs. 3F–H). The percentage of time spent (t = −2.327, p < 0.05) (Supplementary Figs. 3G–H) and percentage of entries in open arms (t = −1.279, p = 0.222) (Supplementary Fig. 3J) were also increased after diazepam treatment in the EPM. But entries in close arms (t = −0.112, p = 0.913) (Supplementary Fig. 3L) were not changed. Diazepam treatment did not change any indicators of self-grooming behaviors in CFA mice compared with saline injection in CFA mice (Supplementary Fig. 3M–O). Diazepam treatment showed a similar anxiolytic effect between the ASD model and stress model, which indicates that stereotyped self-grooming in KO mice was partially due to increased anxiety-like behavior. However, the distinct outcomes in these three anxiety-related mouse models suggested that the underlying pathology of stereotyped self-grooming was primarily contributed to other factors.
Fig. 3.
Repetitive self-grooming in the ASD model and stress model was partially and differently reduced when anxiety-like behaviors were relieved by diazepam.
A, Schematic diagram of the behavioral testing protocol of diazepam treatment in Shank3B KO mice. B-E, Trajectory trials and OFT results in Shank3B KO mice after diazepam treatment. B-D, Diazepam treatment significantly increased the percentage of time spent in the center area and entries into the center area in KO mice (unpaired t-test, KO + S group n = 6 mice, KO + D group n = 9 mice). E, Diazepam treatment did not increase the total distance traveled by KO mice in the OFT (unpaired t-test). F-G, Trajectory trials and results of EPM in Shank3B KO mice after diazepam treatment G. There was a tendency to increase the percentage of time spent in the open arms in EPM after diazepam treatment (unpaired t-test). H-J, Total grooming time (unpaired t-test), number of grooming bouts (unpaired t-test), and the mean grooming duration were all significantly reduced after diazepam treatment (Mann-Whitney U test). K, Schematic diagram of the behavior test protocol of diazepam treatment in the restraint stress-induced anxiety model. L-O, Trajectory trials and OFT results in restraint mice after diazepam treatment. M-N, Diazepam treatment significantly increased the percentage of time spent in the center area and entries into the center area in restraint mice (unpaired t-test, Restraint + S group n = 8 mice, Restraint + D group n = 6 mice). O, Diazepam treatment increased the total distance traveled by restraint mice in the OFT (Two-tailed unpaired separate variance estimation t-test). P-Q, Trajectory trials and results of OFT in restraint mice after diazepam treatment. Q, Time percentage spent in the open arms was increased in EPM after diazepam treatment (Mann-Whitney U test). R-T, Total grooming time (Mann-Whitney U test) and number of grooming bouts (unpaired t-test) were significantly reduced after diazepam treatment, but the mean grooming duration (unpaired t-test) was not altered. Abbreviation: S=Saline, D = Diazepam. Data are shown as the means ± SEM. *p < 0.05, **p < 0.01.
3.4. Diazepam showed diverse effects on the microstructure of stereotyped self-grooming in the ASD model and stress model
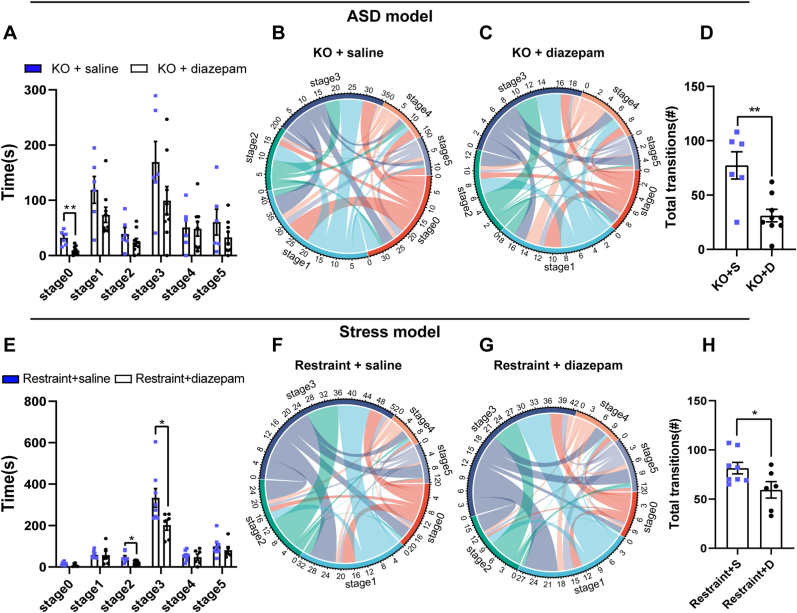
Diazepam reduced stereotyped self-grooming duration. Therefore, we wondered whether diazepam treatment would affect the distribution and switch pattern of self-grooming microstructure in the three anxiety-related mouse models. We found that diazepam treatment only reduced stage 0 (t = 4.221, p < 0.01; KO + S group n = 6 mice, KO + D group n = 9 mice), but not the other stages, of self-grooming microstructure in Shank3B KO mice (Fig. 4A). In the KO treated with saline group, stage 0, stage 1 and stage 3 were the major centers of the switching pattern of the self-grooming microstructure. The switching pattern included sequence and reverse order from other stages to these three stages. However, Stage 1, stage 2, and stage 3 were the center of switching in the microstructure (Fig. 4B–C) in the KO mice treated with diazepam, the switching frequency was reduced (t = 3.740, p < 0.01) (Fig. 4D). In the acute restraint stress mouse model, diazepam treatment reduced stage 2 (t = 2.214, p < 0.05; Restraint + S group n = 8 mice, Restraint + D group n = 6 mice) and stage 3 (Z = −2.324, p < 0.05) of the self-grooming microstructure compared to saline injection (Fig. 4E). Stage 3 remained the core in the restraint mice treated with diazepam and saline. Stage 1 also showed some strong switching in both groups (Fig. 4F–G) and the total transitions of switch were reduced in restraint mice treated with diazepam (t = 2.247, p < 0.05) (Fig. 4H). The distributions of the self-grooming microstructure in the CFA pain model were similar between the CFA mice treated with diazepam and saline (Supplementary Figs. 2E–H; CFA + S group n = 6 mice, CFA + D group n = 5 mice). Taken together, these results indicated that diazepam treatment did not correct the microstructure of the self-grooming in Shank3B KO mice but partially corrected it in the restraint stress-induced anxiety model.
Fig. 4.
Diazepam produced diverse effects on the microstructure of repetitive self-grooming in the ASD model and stress model.
A, Diazepam treatment did not change the self-grooming microstructure and only reduced stage 0 (unpaired t-test, KO + S group n = 6 mice, KO + D group n = 9 mice). B-D, The switching frequency was reduced in the KO mice treated with diazepam (unpaired t-test). E, Diazepam treatment reduced the stage 2 and stage 3 of the self-grooming microstructure in restraint mice compared to saline injection (unpaired t-test, Restraint + S group n = 8 mice, Restraint + D group n = 6 mice). F–H, The switching frequency was reduced in restraint mice treated with diazepam (unpaired t-test). Data are shown as the means ± SEM. *p < 0.05, **p < 0.01.
3.5. The ASD model and stress model shared some commonly activated brain regions but exhibited a few differentiated areas during stereotyped self-grooming behavior
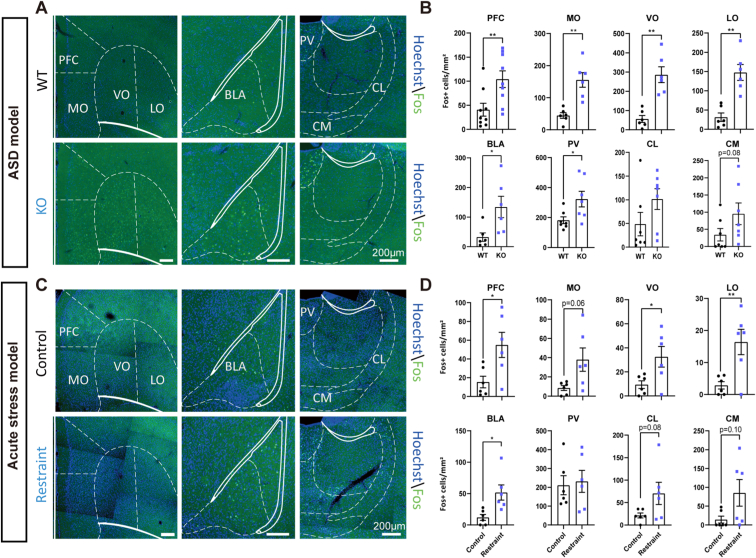
We wondered whether different brain regions dominated repetitive grooming behavior in the ASD model and stress model. To answer this question, we used the c-Fos strategy to explore the functional neural circuits underlying repetitive grooming behavior (Fig. 5A, C). The orbitofrontal cortex (OFC) is involved in grooming behavior (Burguiere et al., 2013). Therefore, we first explored whether OFC subregions, including the MO (medial orbital cortex), VO (ventral orbital cortex), and LO (lateral orbital cortex), were involved in this process. Consistent with previous research (Burguiere et al., 2013; Chen et al., 2021), we found that c-Fos in the OFC was increased in both models, and the change in the subregions was similar, except there was only an increasing tendency in the MO in the stress model (ASD, MO: t = −4.550, p < 0.01; VO: t = −5.113, p < 0.01; LO: t = −4.974, p < 0.01; Restraint, MO: t = −2.373, p = 0.059; VO: t = −2.525, p < 0.05; LO: t = −3.289, p < 0.01) (Fig. 5B, D). Increasing pieces of evidence demonstrated that the prefrontal cortex (PFC) and basolateral amygdala (BLA) participated in anxiety (Hare and Duman, 2020). We discovered that the PFC and BLA had increased expression of c-Fos (ASD, PFC: Z = −2.605, p < 0.01; BLA: t = −2.616, p < 0.05; Restraint, PFC: t = −2.678, p < 0.05; BLA: t = −3.052, p < 0.05). However, the paraventricular thalamic nucleus (PV) was activated in the Shank3B KO ASD model but not in the stress model (ASD, PV: t = −2.491, p < 0.05; Restraint, PV: t = −0.266, p = 0.795). Other regions, such as the centrolateral thalamic nucleus central (CL) and medial thalamic nucleus (CM), had a similar tendency of increased c-Fos in both models (ASD, CL: Z = −1.597, p = 0.110; CM: Z = −1.733, p = 0.083; Restraint, CL: t = −1.942, p = 0.081; CM: Z = −1.660, p = 0.097) (Fig. 5B, D). Grooming behavior involves a series of movements that need adjustment by perception, and we found that the motor cortex (M2) and the somatosensory cortex (S1) were activated in these two models. The anterior cingulate cortex (ACC) was also activated in these two models (ASD, ACC: Z = −2.722, p < 0.01; M2: t = −4.380, p < 0.01; S1Tr: Z = −1.922, p = 0.055; S1BF: t = −4.253, p < 0.01; Restraint, ACC: Z = −2.562, p < 0.05; M2: t = −4.415, p < 0.01; S1Tr: Z = −2.478, p < 0.05; S1BF: Z = −2.478, p < 0.05) (Supplementary Fig. 4).
Fig. 5.
The ASD model and stress model shared some commonly activated brain regions but also exhibited a few differentiated areas during repetitive self-grooming behavior.
A-B, We used the c-Fos strategy to explore the functional anatomical mapping of neuronal circuits underlying grooming behavior. c-Fos was increased in the prefrontal cortex (PFC) (Mann-Whitney U test) and orbitofrontal cortex (OFC), including the medial orbital cortex (MO) (unpaired t-test), ventral orbital cortex (VO) (unpaired t-test), lateral orbital cortex (LO) (unpaired t-test), basolateral amygdala (BLA) (unpaired t-test), and thalamic nucleus (PV) (Two-tailed unpaired separate variance estimation t-test), but not in the thalamic nucleus central (CL) (Mann-Whitney U test) or medial thalamic nucleus (CM) (Mann-Whitney U test) in Shank3B KO autistic mice (n represents slices from 4 mice per group). C-D, c-Fos was increased in the PFC, VO (unpaired t-test), LO (Two-tailed unpaired separate variance estimation t-test), and BLA but not in the MO (Two-tailed unpaired separate variance estimation t-test), PV, CL, and CM (unpaired t-test) in the stress-induced anxiety model (n represents slices from 3 mice per group). Data are shown as the means ± SEM. *p < 0.05, **p < 0.01.
Together, there were wide commonly activated brain regions in the Shank3B KO ASD model and stress model, but certain differentiated areas were also activated. For example, the PFC, BLA, M2, S1, and ACC were activated, but the CM and CL were not activated in either model. The PV and MO were only activated in the Shank3B KO ASD model but not in the stress model.
4. Discussion
Self-grooming is an innate behavioral repertoire for rodent hygiene and care for the body surface (Spruijt et al., 1992) and is an important endophenotype for assessing treatment effects and exploring the neural mechanisms in various psychiatric disorders (Burket et al., 2021; Pinhal et al., 2018; Reis-Silva et al., 2019). As an autistic model, the Shank3B KO mouse exhibits robust self-grooming, which is considered a repetitive stereotyped behavior (Peca et al., 2011). However, self-grooming is also sensitive to alterations in the environment and stress, which suggests that anxiety increases self-grooming (Dunn et al., 1987; Kalueff and Tuohimaa, 2005). Some pieces of evidence have shown that high anxiety is accompanied by more stereotyped behavior in ASD patients and animal models (Baribeau et al., 2020; Peca et al., 2011; Rodgers et al., 2012; Wang et al., 2017a). Our present study verified that the Shank3B KO autistic mouse model (ASD model), acute restraint stress model (stress model), and CFA-induced chronic inflammatory pain model (pain model) all induced increased anxiety. However, the self-grooming behaviors were different between these three models. The ASD model and stress model showed increased self-grooming, but the pain model did not alter this behavior. And even the Shank3B KO ASD model and stress model also showed some differences in the self-grooming parameters. The increased total grooming duration of Shank3B KO mice was primarily due to an increase in the mean grooming duration, but the increase in the restraint stress-induced anxiety model was due to the sum of the number of grooming bouts and mean grooming duration. This difference suggests that the correlation between anxiety and stereotyped behavior may rely on multiple factors, such as the environment and genetics. For example, the acute restraint paradigm produces obvious stress and apparent fur moistening. The latter condition is also an important environmental factor that causes increased self-grooming (van Erp et al., 1994). The excessive self-grooming of Shank3B KO mice may be primarily caused by genetic deficits.
Self-grooming is a cephalo-caudal progression and consists of several stages, or grooming microstructure (Kalueff et al., 2016). Compared with the general parameters of self-grooming, such as total grooming time and grooming bouts, the grooming microstructure analysis may provide distinct information for understanding stereotyped behavior and related anxiety (Kalueff et al., 2007; Kalueff and Tuohimaa, 2004). Although we found both the ASD model and stress model showed increased total grooming time, there was a great difference in the grooming microstructure of the ASD model and the stress model compared to their control mice. We found that the grooming time of Shank3B KO mice primarily existed in stage 2 and stage 3, and the restraint stress-induced anxiety model primarily existed in stage 3, stage 4, and stage 5. The grooming transitions of the three models all exhibited distinct patterns. However, we did not find a significant difference in all stages or the stage transitions of self-grooming between the pain model and control mice, although the pain model showed increased anxiety-like behavior. It suggested that the microstructure stages and their transitions of stereotyped self-grooming may be dependent on not only the stress but also other disease conditions (Kalueff and Tuohimaa, 2004; Pires et al., 2013). Our speculation was further supported by the finding that diazepam treatment partially rescued the grooming microstructures of the stress model but not the ASD model. Our recent work found that excitation of the ACC relieved anxiety-like behavior but not self-grooming behavior in Shank3B KO mice (Guo et al., 2019). Taken together, our present work demonstrated that Shank3B gene mutations may cause two separate but interrelated, neural circuit deficits for modulating anxiety and repetitive stereotyped behaviors.
The benzodiazepine drug diazepam has a good anxiolytic effect through enhancing GABAergic tone in clinical and basic research (Fraga et al., 2018; Gao et al., 2018). Previous research reported that a single dose of diazepam effectively reduced the level of anxiety in mice (Aragao et al., 2006). And several drugs increasing GABAergic tone was found to decrease the grooming frequency of the rats in the OFT (Barros et al., 1994). Since the GABAergic system is a key modulator of stress and anxiety-related behaviors in rodents (Kalueff and Nutt, 1996), diazepam may suppress self-grooming through the anxiolytic mechanism. Our present work found that diazepam effectively reduced anxiety-like behaviors in all three models. Notably, the administration of diazepam simultaneously reduced the total grooming duration of the Shank3B KO mice and the stress model, which indicated that anxiety and stereotyped self-grooming correlated with each other in these two models. However, there were some detailed differences between these two models, especially the grooming microstructure. The number of grooming bouts and mean grooming duration of Shank3B KO mice were reduced, but only the number of grooming bouts was reduced in the stress model. This result further supported distinct mechanisms of increased self-grooming behavior between these two models. Self-grooming reflects complex values for understanding the work process of the brain, at least including the hierarchical motor control and emotion state. As an autistic model, the Shank3B KO mouse exhibits robust self-grooming, which is considered a repetitive stereotyped behavior (Peca et al., 2011). Our previous work found the striatum, a brain region involved in motor sequencing, contributed to the repetitive self-grooming in this mouse model (Wang et al., 2017b). However, self-grooming is also sensitive to alterations in the environment and stress, which suggests that emotion may affect self-grooming (Dunn et al., 1987; Kalueff and Tuohimaa, 2005). Thus, we further used the c-Fos strategy to compare the brain regions that governed grooming behavior in the two models, we found that most of the brain regions activated by the two models overlapped. The activated brain regions were related to anxiety, such as the ACC, PFC, and BLA (Guo et al., 2018, 2019; Hare and Duman, 2020), and perception and movement, such as S1 and M2. However, there were some differences between the two models. For example, the PV and MO were activated in Shank3B KO mice but not the stress model mice. These differences may be produced by distinct pathogenesis (stress and Shank3B mutation) triggering various circuit changes related to self-grooming behavior or other unrelated behaviors.
Our present work still has some limitations. Firstly, the self-grooming behavior was recorded from a side-view camera which may miss some information when the mouse turns its back on the camera in our experiments. It still is a problem even we record the self-grooming from the top of the chamber. And our analysis of self-grooming still relies on manual analysis. One resolution in the future is to use a 3D animal motion-capture system and machine learning analysis framework to explore this type of spontaneous behavior (Huang et al., 2021), which can provide more intact information for understanding self-grooming and anxiety (Liu et al., 2021). It also can save the experimenter a lot of time and energy. Secondly, when we used the c-Fos strategy to map the activation brain regions related to the self-grooming in the ASDs models and the stress models, we cannot fully exclude the possibility that the difference of activation between these two models was caused by other behavior unrelated to the self-grooming. For example, mice might spend lots of time in exploring environment, which intertwine with grooming activity, making the results unintelligible. Behavior-related neuronal activities recording with the genetically encoded calcium indicator in the specific brain regions may provide more detailed information for revealing the precise relationship between self-grooming and anxiety in the future.
In summary, our results demonstrate that the Shank3B KO ASD model, the stress model, and the pain model all showed increased anxiety. However, the ASD model and stress model, but not the pain model, produced obvious stereotyped self-grooming. The grooming microstructure of the three models exhibited distinct patterns. The anxiolytic medicine diazepam reduced the total grooming time, but not the grooming microstructure, in the ASD model. Our findings demonstrated that stereotyped self-grooming and anxiety displayed entangled interactions in distinct disease models, self-grooming can be caused by anxiety but there were far more factors such as gene mutant, which may help elucidate the neural mechanism and develop potential treatments for stereotyped behaviors and anxiety.
CRediT authorship contribution statement
Haiying Liu: Conceptualization, Methodology, Investigation, Formal analysis, Writing – original draft. Xin Huang: Methodology, Investigation, Formal analysis, Writing – review & editing. Jinwei Xu: Formal analysis, Investigation, Methodology. Honghui Mao: Formal analysis, Formal analysis. Yaohao Li: Formal analysis, Investigation, Methodology. Keke Ren: Formal analysis. Guaiguai Ma: Formal analysis. Qian Xue: Formal analysis. Huiren Tao: revised manuscript discussion and suggestion. Shengxi Wu: Conceptualization, Writing – review & editing. Wenting Wang: Conceptualization, Supervision, Writing – review & editing.
Declaration of competing interest
The authors have no conflicts of interest.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (81771476 and 82071536 to Wenting Wang, 81730035 to Shengxi Wu), Shaanxi Provincial Key Research and Development Program (Grant No:2020ZDLSF01–09 to Shengxi Wu), Shaanxi Key Research and Development Program (Grant No. 2020SF-127 to Qian Xue), and CAS Key Laboratory of Brain Connectome and Manipulation (2019DP173024 to Wenting Wang). We thank American Journal Experts for English language editing (certificate verification code: 151E-7FF6–9CB8–B6D8-6D0B).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ynstr.2021.100417.
Contributor Information
Shengxi Wu, Email: shengxi@fmmu.edu.cn.
Wenting Wang, Email: wwt0657@fmmu.edu.cn.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- Aragao G.F., Carneiro L.M., Junior A.P., Vieira L.C., Bandeira P.N., Lemos T.L., Viana G.S. A possible mechanism for anxiolytic and antidepressant effects of alpha- and beta-amyrin from Protium heptaphyllum (Aubl.) March. Pharmacol. Biochem. Behav. 2006;85:827–834. doi: 10.1016/j.pbb.2006.11.019. [DOI] [PubMed] [Google Scholar]
- Arakawa H. Implication of the social function of excessive self-grooming behavior in BTBR T(+)ltpr3(tf)/J mice as an idiopathic model of autism. Physiol. Behav. 2021;237 doi: 10.1016/j.physbeh.2021.113432. [DOI] [PubMed] [Google Scholar]
- Askari-Zahabi K., Abbasnejad M., Kooshki R., Esmaeili-Mahani S. Orexin one receptors within the basolateral amygdala are involved in the modulation of cognitive deficits associated with a migraine-like state in rats. Neurol. Res. 2021:1–11. doi: 10.1080/01616412.2021.1949687. 10.1080/01616412.2021.1949687. [DOI] [PubMed] [Google Scholar]
- Baribeau D.A., Vigod S., Pullenayegum E., Kerns C.M., Mirenda P., Smith I.M., Vaillancourt T., Volden J., Waddell C., Zwaigenbaum L., Bennett T., Duku E., Elsabbagh M., Georgiades S., Ungar W.J., Zaidman-Zait A., Szatmari P. Repetitive behavior severity as an early indicator of risk for elevated anxiety symptoms in autism spectrum disorder. J. Am. Acad. Child Adolesc. Psychiatry. 2020;59:890–899. doi: 10.1016/j.jaac.2019.08.478. e893. [DOI] [PubMed] [Google Scholar]
- Baribeau D.A., Vigod S., Pullenayegum E., Kerns C.M., Mirenda P., Smith I.M., Vaillancourt T., Volden J., Waddell C., Zwaigenbaum L., Bennett T., Duku E., Elsabbagh M., Georgiades S., Ungar W.J., Zaidman Zait A., Szatmari P. Co-occurring trajectories of anxiety and insistence on sameness behaviour in autism spectrum disorder. Br. J. Psychiatry. 2021;218:20–27. doi: 10.1192/bjp.2020.127. [DOI] [PubMed] [Google Scholar]
- Barros H.M., Tannhauser S.L., Tannhauser M.A., Tannhauser M. The effects of GABAergic drugs on grooming behaviour in the open field. Pharmacol. Toxicol. 1994;74:339–344. doi: 10.1111/j.1600-0773.1994.tb01370.x. [DOI] [PubMed] [Google Scholar]
- Burguiere E., Monteiro P., Feng G., Graybiel A.M. Optogenetic stimulation of lateral orbitofronto-striatal pathway suppresses compulsive behaviors. Science. 2013;340:1243–1246. doi: 10.1126/science.1232380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burket J.A., Matar M., Fesshaye A., Pickle J.C., Britten R.A. Exposure to low (</=10 cGy) doses of 4He particles leads to increased social withdrawal and loss of executive function performance. Radiat. Res. 2021 doi: 10.1667/RADE-20-00251.1. 10.1667/RADE-20-00251.1. [DOI] [PubMed] [Google Scholar]
- Chen X., Yue J., Luo Y., Huang L., Li B., Wen S. Distinct behavioral traits and associated brain regions in mouse models for obsessive-compulsive disorder. Behav. Brain Funct. 2021;17:4. doi: 10.1186/s12993-021-00177-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choleris E., Thomas A.W., Kavaliers M., Prato F.S. A detailed ethological analysis of the mouse open field test: effects of diazepam, chlordiazepoxide and an extremely low frequency pulsed magnetic field. Neurosci. Biobehav. Rev. 2001;25:235–260. doi: 10.1016/s0149-7634(01)00011-2. [DOI] [PubMed] [Google Scholar]
- Cruz A.P., Frei F., Graeff F.G. Ethopharmacological analysis of rat behavior on the elevated plus-maze. Pharmacol. Biochem. Behav. 1994;49:171–176. doi: 10.1016/0091-3057(94)90472-3. [DOI] [PubMed] [Google Scholar]
- Daviu N., Bruchas M.R., Moghaddam B., Sandi C., Beyeler A. Neurobiological links between stress and anxiety. Neurobiol Stress. 2019;11 doi: 10.1016/j.ynstr.2019.100191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn A.J., Berridge C.W., Lai Y.I., Yachabach T.L. CRF-induced excessive grooming behavior in rats and mice. Peptides. 1987;8:841–844. doi: 10.1016/0196-9781(87)90069-6. [DOI] [PubMed] [Google Scholar]
- Estanislau C., Díaz-Morán S., Cañete T., Blázquez G., Tobeña A., Fernández-Teruel A. Context-dependent differences in grooming behavior among the NIH heterogeneous stock and the Roman high- and low-avoidance rats. Neurosci. Res. 2013;77:187–201. doi: 10.1016/j.neures.2013.09.012. [DOI] [PubMed] [Google Scholar]
- Fraga D.B., Olescowicz G., Moretti M., Siteneski A., Tavares M.K., Azevedo D., Colla A.R.S., Rodrigues A.L.S. Anxiolytic effects of ascorbic acid and ketamine in mice. J. Psychiatr. Res. 2018;100:16–23. doi: 10.1016/j.jpsychires.2018.02.006. [DOI] [PubMed] [Google Scholar]
- Friard O., Boris Gamba M. vol. 7. 2016. pp. 1325–1330. (A Free, Versatile Open-Source Event-Logging Software for Video/audio Coding and Live Observations). [DOI] [Google Scholar]
- Gao G., Qian M.H., Ji C., Yao M. The analysis of the effect of psycho-intervention combined with diazepam on patients with sudden sensorineural hearing loss and anxiety. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2018;32:753–757. doi: 10.13201/j.issn.1001-1781.2018.10.007. [DOI] [PubMed] [Google Scholar]
- Garcia-Keller C., Carter J.S., Kruyer A., Kearns A.M., Hopkins J.L., Hodebourg R., Kalivas P.W., Reichel C.M. Behavioral and accumbens synaptic plasticity induced by cues associated with restraint stress. Neuropsychopharmacology. 2021 doi: 10.1038/s41386-021-01074-7. 10.1038/s41386-021-01074-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotham K., Bishop S.L., Hus V., Huerta M., Lund S., Buja A., Krieger A., Lord C. Exploring the relationship between anxiety and insistence on sameness in autism spectrum disorders. Autism Res. 2013;6:33–41. doi: 10.1002/aur.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo B., Chen J., Chen Q., Ren K., Feng D., Mao H., Yao H., Yang J., Liu H., Liu Y., Jia F., Qi C., Lynn-Jones T., Hu H., Fu Z., Feng G., Wang W., Wu S. Anterior cingulate cortex dysfunction underlies social deficits in Shank3 mutant mice. Nat. Neurosci. 2019;22:1223–1234. doi: 10.1038/s41593-019-0445-9. [DOI] [PubMed] [Google Scholar]
- Guo B., Wang J., Yao H., Ren K., Chen J., Yang J., Cai G., Liu H., Fan Y., Wang W., Wu S. Chronic inflammatory pain impairs mGluR5-mediated depolarization-induced suppression of excitation in the anterior cingulate cortex. Cerebr. Cortex. 2018;28:2118–2130. doi: 10.1093/cercor/bhx117. [DOI] [PubMed] [Google Scholar]
- Hare B.D., Duman R.S. Prefrontal cortex circuits in depression and anxiety: contribution of discrete neuronal populations and target regions. Mol. Psychiatr. 2020;25:2742–2758. doi: 10.1038/s41380-020-0685-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang K., Han Y., Chen K., Pan H., Zhao G., Yi W., Li X., Liu S., Wei P., Wang L. A hierarchical 3D-motion learning framework for animal spontaneous behavior mapping. Nat. Commun. 2021;12:2784. doi: 10.1038/s41467-021-22970-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaworski J., Kalita K., Knapska E. c-Fos and neuronal plasticity: the aftermath of Kaczmarek's theory. Acta Neurobiol. Exp. 2018;78:287–296. doi: 10.21307/ane2018027. [DOI] [PubMed] [Google Scholar]
- Kalinina T.S., Shimshirt A.A., Volkova A.V., Korolev A.O., Voronina T.A. Anxiolytic effects of diazepam and afobazole on the anxiety response evoked by gaba(a) receptor blockade in wistar rats and inbred mice of bALB/C and c57bi/6 strains. Eksp. Klin. Farmakol. 2016;79:3–7. [PubMed] [Google Scholar]
- Kalueff A., Nutt D.J. Role of GABA in memory and anxiety. Depress. Anxiety. 1996;4:100–110. doi: 10.1002/(sici)1520-6394. (1996)4:3<100::Aid-da2>3.0.Co;2-k. [DOI] [PubMed] [Google Scholar]
- Kalueff A.V., Aldridge J.W., LaPorte J.L., Murphy D.L., Tuohimaa P. Analyzing grooming microstructure in neurobehavioral experiments. Nat. Protoc. 2007;2:2538–2544. doi: 10.1038/nprot.2007.367. [DOI] [PubMed] [Google Scholar]
- Kalueff A.V., Stewart A.M., Song C., Berridge K.C., Graybiel A.M., Fentress J.C. Neurobiology of rodent self-grooming and its value for translational neuroscience. Nat. Rev. Neurosci. 2016;17:45–59. doi: 10.1038/nrn.2015.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalueff A.V., Tuohimaa P. Grooming analysis algorithm for neurobehavioural stress research. Brain Res Brain Res Protoc. 2004;13:151–158. doi: 10.1016/j.brainresprot.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Kalueff A.V., Tuohimaa P. The grooming analysis algorithm discriminates between different levels of anxiety in rats: potential utility for neurobehavioural stress research. J. Neurosci. Methods. 2005;143:169–177. doi: 10.1016/j.jneumeth.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Kraeuter A.K., Guest P.C., Sarnyai Z. The elevated plus maze test for measuring anxiety-like behavior in rodents. Methods Mol. Biol. 2019;1916:69–74. doi: 10.1007/978-1-4939-8994-2_4. [DOI] [PubMed] [Google Scholar]
- Kraeuter A.K., Guest P.C., Sarnyai Z. The open field test for measuring locomotor activity and anxiety-like behavior. Methods Mol. Biol. 2019;1916:99–103. doi: 10.1007/978-1-4939-8994-2_9. [DOI] [PubMed] [Google Scholar]
- Lai M.C., Lombardo M.V., Baron-Cohen S. Autism. Lancet. 2014;383:896–910. doi: 10.1016/S0140-6736(13)61539-1. [DOI] [PubMed] [Google Scholar]
- Liu N., Han Y., Ding H., Huang K., Wei P., Wang L. Objective and comprehensive re-evaluation of anxiety-like behaviors in mice using the Behavior Atlas. Biochem. Biophys. Res. Commun. 2021;559:1–7. doi: 10.1016/j.bbrc.2021.03.125. [DOI] [PubMed] [Google Scholar]
- Meyza K.Z., Defensor E.B., Jensen A.L., Corley M.J., Pearson B.L., Pobbe R.L., Bolivar V.J., Blanchard D.C., Blanchard R.J. The BTBR T+ tf/J mouse model for autism spectrum disorders-in search of biomarkers. Behav. Brain Res. 2013;251:25–34. doi: 10.1016/j.bbr.2012.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody T.W., Merali Z., Crawley J.N. The effects of anxiolytics and other agents on rat grooming behavior. Ann. N. Y. Acad. Sci. 1988;525:281–290. doi: 10.1111/j.1749-6632.1988.tb38613.x. [DOI] [PubMed] [Google Scholar]
- Mosaffa S., Ahmadi H., Khakpai F., Ebrahimi-Ghiri M., Zarrindast M.R. Synergistic antidepressant- and anxiolytic-like effects of harmaline along with cinanserin in acute restraint stress-treated mice. Psychopharmacology (Berl) 2021;238:259–269. doi: 10.1007/s00213-020-05679-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu M.D., Geng H.Y., Rong K.L., Peng R.C., Wang S.T., Geng L.T., Qian Z.M., Yung W.H., Ke Y. A limbic circuitry involved in emotional stress-induced grooming. Nat. Commun. 2020;11:2261. doi: 10.1038/s41467-020-16203-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peca J., Feliciano C., Ting J.T., Wang W., Wells M.F., Venkatraman T.N., Lascola C.D., Fu Z., Feng G. Shank3 mutant mice display autistic-like behaviours and striatal dysfunction. Nature. 2011;472:437–442. doi: 10.1038/nature09965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertusa A., Fullana M.A., Singh S., Alonso P., Menchón J.M., Mataix-Cols D. Compulsive hoarding: OCD symptom, distinct clinical syndrome, or both? Am. J. Psychiatr. 2008;165:1289–1298. doi: 10.1176/appi.ajp.2008.07111730. [DOI] [PubMed] [Google Scholar]
- Pinhal C.M., van den Boom B.J.G., Santana-Kragelund F., Fellinger L., Bech P., Hamelink R., Feng G., Willuhn I., Feenstra M.G.P., Denys D. Differential effects of deep brain stimulation of the internal capsule and the striatum on excessive grooming in Sapap3 mutant mice. Biol. Psychiatr. 2018;84:917–925. doi: 10.1016/j.biopsych.2018.05.011. [DOI] [PubMed] [Google Scholar]
- Pires G.N., Tufik S., Andersen M.L. Grooming analysis algorithm: use in the relationship between sleep deprivation and anxiety-like behavior. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2013;41:6–10. doi: 10.1016/j.pnpbp.2012.11.006. [DOI] [PubMed] [Google Scholar]
- Rapp J.T., Vollmer T.R. Stereotypy I: a review of behavioral assessment and treatment. Res. Dev. Disabil. 2005;26:527–547. doi: 10.1016/j.ridd.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Reis-Silva T.M., Sandini T.M., Calefi A.S., Orlando B.C.G., Moreira N., Lima A.P.N., Florio J.C., Queiroz-Hazarbassanov N.G.T., Bernardi M.M. Stress resilience evidenced by grooming behaviour and dopamine levels in male mice selected for high and low immobility using the tail suspension test. Eur. J. Neurosci. 2019;50:2942–2954. doi: 10.1111/ejn.14409. [DOI] [PubMed] [Google Scholar]
- Renno P., Wood J.J. Discriminant and convergent validity of the anxiety construct in children with autism spectrum disorders. J. Autism Dev. Disord. 2013;43:2135–2146. doi: 10.1007/s10803-013-1767-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers J., Glod M., Connolly B., McConachie H. The relationship between anxiety and repetitive behaviours in autism spectrum disorder. J. Autism Dev. Disord. 2012;42:2404–2409. doi: 10.1007/s10803-012-1531-y. [DOI] [PubMed] [Google Scholar]
- Spruijt B.M., van Hooff J.A., Gispen W.H. Ethology and neurobiology of grooming behavior. Physiol. Rev. 1992;72:825–852. doi: 10.1152/physrev.1992.72.3.825. [DOI] [PubMed] [Google Scholar]
- Taylor B.A., Hoch H., Weissman M. The analysis and treatment of vocal stereotypy in a child with autism. Behav. Interv. 2005;20:239–253. doi: 10.1002/bin.200. [DOI] [Google Scholar]
- Uljarević M., Frazier T.W., Jo B., Billingham W.D., Cooper M.N., Youngstrom E.A., Scahill L., Hardan A.Y. Big data approach to characterize restricted and repetitive behaviors in autism. J. Am. Acad. Child Adolesc. Psychiatry. 2021 doi: 10.1016/j.jaac.2021.08.006. doi.org/10.1016/j.jaac.2021.08.006. [DOI] [PubMed] [Google Scholar]
- van Erp A.M., Kruk M.R., Meelis W., Willekens-Bramer D.C. Effect of environmental stressors on time course, variability and form of self-grooming in the rat: handling, social contact, defeat, novelty, restraint and Fur moistening. Behav. Brain Res. 1994;65:47–55. doi: 10.1016/0166-4328(94)90072-8. [DOI] [PubMed] [Google Scholar]
- van Steensel F.J., Bögels S.M., Perrin S. Anxiety disorders in children and adolescents with autistic spectrum disorders: a meta-analysis. Clin. Child Fam. Psychol. Rev. 2011;14:302–317. doi: 10.1007/s10567-011-0097-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B., Zheng Y., Shi H., Du X., Zhang Y., Wei B., Luo M., Wang H., Wu X., Hua X., Sun M., Xu X. Zfp462 deficiency causes anxiety-like behaviors with excessive self-grooming in mice. Gene Brain Behav. 2017;16:296–307. doi: 10.1111/gbb.12339. [DOI] [PubMed] [Google Scholar]
- Wang W., Li C., Chen Q., van der Goes M.S., Hawrot J., Yao A.Y., Gao X., Lu C., Zang Y., Zhang Q., Lyman K., Wang D., Guo B., Wu S., Gerfen C.R., Fu Z., Feng G. Striatopallidal dysfunction underlies repetitive behavior in Shank3-deficient model of autism. J. Clin. Invest. 2017;127:1978–1990. doi: 10.1172/JCI87997. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.