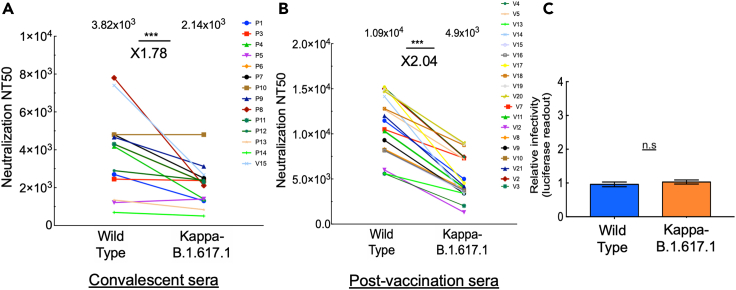
Figure 1.
Post-vaccination neutralization sensitivity and infectivity of the Kappa-B.1.617.1 SARS-CoV-2 variant
(A and B) Convalescent or post-vaccination sera neutralize Kappa-B.1.6171.1 SARS-CoV-2 pseudoviruses; neutralization assays were performed by transducing HEK-ACE2 cells with pseudoviruses displaying spike protein of either wild-type SARS-CoV-2 or its Kappa-B.1.617.1 variant, in the presence of increasing dilutions of convalescent (A) or post-vaccination (B) sera. At 48 h post-transduction, cells were harvested and their luciferase readings were monitored. Neutralizing potency was calculated at increased serial dilutions, relative to transduced cells with no sera added. Neutralization, NT50, is defined as the inverse dilution that achieved 50% neutralization. Results are the average of two independent biological experiments. Triplicates were performed for each tested serum dilution. Black bars represent geometric mean of NT50 values, indicated at the top. Statistical significance was determined using one-tailed t test; ∗∗∗p<0.001.
(C) Infectivity levels of Kappa-B.1.617.1 SARS-CoV-2 pseudoviruses; pseudoviruses bearing wild-type or B.1.617.1 SARS-CoV-2 spike mutations were used to transduce HEK-ACE2 target cells. Equal viral loads were normalized based on their p24 protein levels. At 48 h post-transduction, cells were harvested and their luciferase readouts were monitored. Bar graphs show mean values ±SD error bars of three independent experiments resulted in non-significance statistical difference.