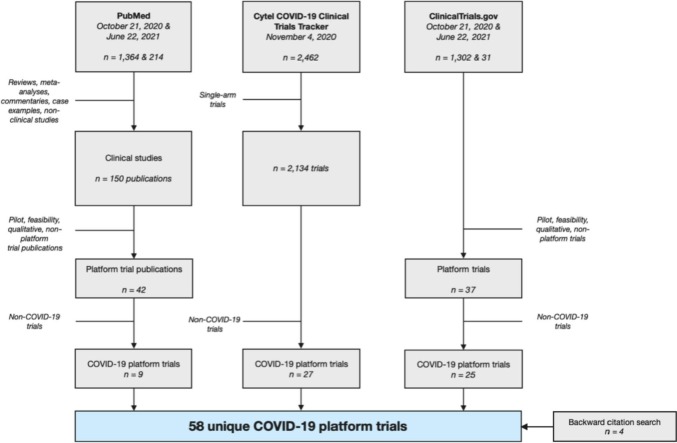
Fig. 1.
PRISMA flowchart for combined search and selection at two time points. We searched ClinicalTrials.gov for master protocols in any indication (October 21, 2020) and COVID-19 (June 22, 2021). We searched PubMed for master protocols in any indication (October 21, 2020 and June 22, 2020). The full Cytel COVID-19 Clinical Trials Tracker dataset was retrieved on November 4th for COVID-19-specific trials of any design. A trial was labeled as platform if it is stated on the trial registration webpage, in a publication, or in the full study protocol that the trial allows for the addition of future arms [3]. A trial was labeled as “potential” platform if the listed sources contained language that suggested but could not confirm the flexibility to add arms, or the trial self-identifies as platform but has no further information.