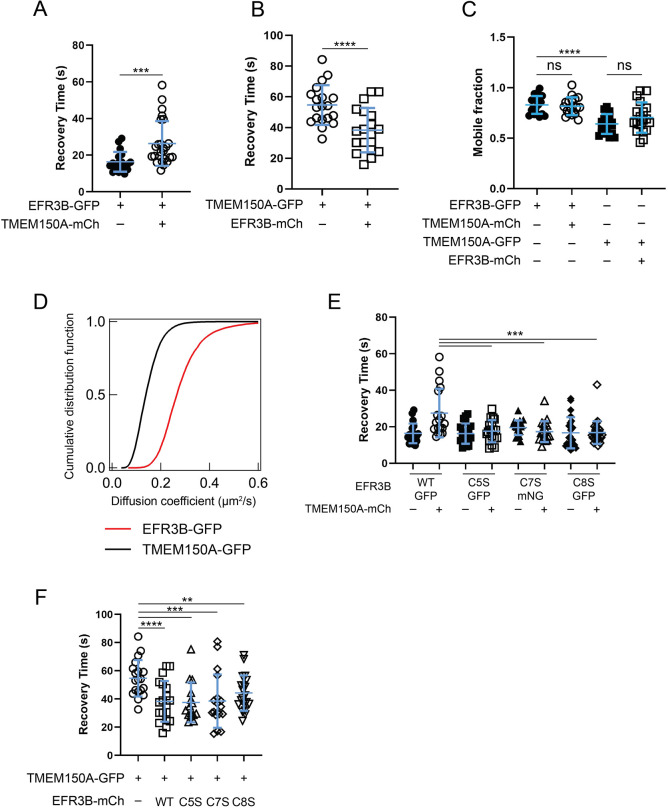
Fig. 2.
EFR3B interacts with TMEM150A, and the membrane dynamics of WT and CxS mutant EFR3B are similar. (A) FRAP recovery times (t1/2) of EFR3B–GFP with or without TMEM150A–mCherry (TMEM150A–mCh). The increase in the recovery time of EFR3B in the presence of TMEM150A suggests an interaction between the two proteins. (B) FRAP recovery times (t1/2) of TMEM150A–GFP with or without EFR3B–mCherry (EFR3B–mCh). Note that the recovery times of co-expressed TMEM150A and EFR3B move closer to each other (compare A and B). (C) Mobile fraction of EFR3B–GFP or TMEM150A–GFP when expressed alone or together as evaluated by FRAP. (D) Diffusion coefficients of EFR3B–GFP and TMEM150A–GFP, expressed separately, as measured by ImFCS. Data are pooled from three independent experiments, n=15–16 cells. (E) Recovery times (t1/2) of WT EFR3B–GFP and individual EFR3B–GFP or EFR3B–mNG CxS mutants with or without TMEM150A–mCherry. Note that the CxS mutations have no effect on the membrane diffusion of EFR3B and are insensitive to the presence of TMEM150A. (F) Recovery time (t1/2) of TMEM150A–GFP expressed alone or in the presence of WT or CxS mutant EFR3B–mCherry. Note that the CxS mutants have the same effect on TMEM150A–GFP recovery time as WT EFR3B. To improve clarity and facilitate comparisons between effects of single or co-expression, the data in A and B are also plotted in E and F, respectively. Data in A–C,E,F are mean±s.d., n=14–27. **P<0.01; ***P<0.005; ****P<0.001; ns, not significant (two-tailed unpaired t-test in A and B; one-way ANOVA with Tukey–Kramer post-hoc test in C,E and F).