ABSTRACT.
Rotavirus is responsible for 26% of diarrheal deaths in Latin America and the Caribbean. Haiti introduced the monovalent rotavirus vaccine in April 2014. The objective of this analysis is to describe the impact of the rotavirus vaccine on hospitalizations among Haitian children younger than 5 years old during the first 5 years after introduction. This analysis includes all children with diarrhea who were enrolled as part of a sentinel surveillance system at two hospitals from May 2013 to April 2019. We compare the proportion of rotavirus-positive specimens in each post-vaccine introduction year to the pre-vaccine period. To account for the potential dilution of the proportion of rotavirus-positive specimens from a waning cholera outbreak, we also analyzed annual trends in the absolute number of positive stools, fit a two-component finite-mixture model to the negative specimens, and fit a negative binomial time series model to the pre-vaccine rotavirus-positive specimens to predict the number of rotavirus diarrhea hospital admissions in the absence of rotavirus vaccination. The overall percentage of rotavirus-positive specimens declined by 22% the first year after introduction, increased by 17% the second year, and declined by 33% to 50% the subsequent 3 years. All sensitivity analyses confirmed an overall decline. We observed a clear annual rotavirus seasonality before and after vaccine introduction, with the greatest activity in December through April, and a biennial pattern, with high sharp peaks and flatter longer periods of increased rotavirus activity in alternating years, consistent with suboptimal vaccination coverage. Overall, our study shows evidence that the introduction of the rotavirus vaccine reduced the burden of severe rotavirus diarrhea.
INTRODUCTION
Rotavirus is a major cause of acute gastroenteritis in children younger than 5 years of age worldwide and was responsible for 26% of diarrheal deaths in Latin America and the Caribbean in 2013.1,2 Rotavirus vaccines were first licensed in 2006 and, after demonstrating substantial reductions in severe rotavirus disease among children in high- and middle-income countries in the Americas and Europe, the WHO recommended all countries introduce rotavirus vaccines into their routine immunization programs in 2009.3,4 More than 100 countries worldwide have introduced rotavirus vaccine to date.5 In countries where rotavirus vaccine is used routinely, all-cause acute gastroenteritis hospitalizations among children younger than 5 years old have been reduced by more than one third, and rotavirus hospitalizations by nearly two thirds.6
In 2012, the Haitian Ministry of Public Health and Population and international partners established Projet de renforcement de sruveillance et epidemiology (PRESEpi), an active, integrated, laboratory-enhanced sentinel surveillance system.7–9 PRESEpi monitors acute gastroenteritis and four other syndromes. The acute gastroenteritis surveillance portion of PRESEpi enrolls cases with acute watery diarrhea, and tests stool specimens for rotavirus and cholera, because both are characterized by acute watery diarrhea.9
Several evaluations published since cholera transmission began have reported a high burden of all-cause diarrheal disease among children younger than 5 years old in Haiti.10,11 Findings from PRESEpi showed that in 2012 to 2013, 15% of children younger than 2 years old hospitalized as a result of diarrhea were positive for rotavirus and 13% were positive for cholera. During the same period, 10% of children 2 to 4 years old were positive for rotavirus and 49% for cholera—a similar distribution to that described in Bangladesh.12,13 The authors of the earlier PRESEpi evaluation attributed this low proportion of rotavirus to inflation of the all-cause acute gastroenteritis denominator by the cholera outbreak, which caused more than 100,000 cases and nearly 1,000 deaths resulting from cholera nationwide in 2012.12 The annual number of cholera cases and case fatality rate have decreased during the past 10 years, from a high of more than 4,000 deaths in 2010 and more than 350,000 cases in 2011 to less than 700 cases and three deaths in 2019.8,14
Haiti introduced the monovalent rotavirus vaccine (Rotarix, GlaxoSmithKline Biologicals, Rixensart, Belgium) in April 2014.9 Rotavirus vaccination coverage among children younger than 12 months old has increased slowly from 40% in 2014 to 73% in 2018, then decreased to 63% in 2019.15 The objective of this analysis is to describe the impact of the rotavirus vaccine on rotavirus hospitalizations among Haitian children younger than 5 years old during the first 5 years after rotavirus vaccine introduction, in the setting of a waning cholera epidemic. To the best of our knowledge, this is the first description of rotavirus vaccine implementation in the context of concurrent rotavirus and cholera transmission.
METHODS
Surveillance methods.
The PRESEpi surveillance procedures and structure have been described in detail previously.9,12 Briefly, PRESEpi enrolls patients of any age hospitalized for one or more of four syndromes: acute gastroenteritis, acute febrile illness, acute respiratory illness, and pneumonia with pleural effusion and meningitis. Since 2012, four initial sentinel hospital sites have participated in the surveillance network. Hopital Universitare de la Paix, a public hospital, and Hopital St. Camille, a private hospital, are located in Port-au-Prince, the capital in Ouest Department. Hopital St. Michel de Jacmel, a private hospital, is located in Jacmel, Sud Est Department; and Hopital St. Nicholas, a public hospital, is located in St. Marc, Artibonite Department. In 2014, two additional sites in Port-au-Prince and, in 2016, 10 more sites in the remaining seven departments of Haiti joined the network. Upon enrollment, a structured questionnaire detailing demographics, history of the illness, and clinical findings at enrollment is administered. Initially, weekly enrollment from each site was limited to only 10 cases of acute gastroenteritis of any age. This practice was changed in 2013, when all cases of acute gastroenteritis in children younger than 5 years were subsequently enrolled. For these patients, a stool specimen was obtained within 48 hours of hospitalization and was transported to the Laboratoire National de Sante Publique for rotavirus antigen detection using Rotaclone (Meridian) enzyme immunoassay testing.
Our analysis includes all children younger than 5 years who were hospitalized and enrolled in PRESEpi from May 2013 through April 2019 at the Hopital St. Nicholas and Hopital St. Camille sentinel sites for acute gastroenteritis (defined as the passage of three or more loose or watery, non-bloody stools in a 24-hour period with or without dehydration) and had a rotavirus test. To optimize the pre- and post-vaccine introduction comparisons, we excluded children enrolled before May 2013, when only the first 10 acute gastroenteritis cases (including individuals 5 years old and older) per week were enrolled. We also excluded children enrolled from the Hopital Universitare de la Paix and Hopital St. Michel de Jacmel sentinel sites because of lengthy disruptions in reporting in 2016, and the 10 sites where surveillance began after the rotavirus vaccine was introduced.
Analytical methods.
We defined surveillance years as May through April of the subsequent calendar year, with May 2013 to April 2014 as the pre-vaccine period. We analyzed the annual total number of stools tested for rotavirus, rotavirus-positive tests, and proportion of stools positive for rotavirus among children younger than 1 year, 1 to 4 years, and all children less than 5 years. We compared the proportion of rotavirus-positive specimens in each post-vaccine introduction year (May 2014–April 2015, May 2015–April 2016, May 2016–April 2017, May 2017–April 2018, and May 2018–April 2019) to that of the pre-vaccine period.
To account for the potential dilution of the proportion of rotavirus-positive specimens among cases of acute gastroenteritis resulting from the ongoing cholera outbreak, we conducted two additional analyses. First, we fit a two-component finite-mixture model using negative binomial distributions to the rotavirus-negative data to estimate the number of cases each year that may be the result of cholera.16,17 The few, non-random children who were tested for cholera were included in the model as “classified.” We included categorical variables for monthly and yearly cholera activity among adults enrolled in PRESEpi as predictors, and the national, annual rate of syndromic cholera per 1,000 people as an offset in the cholera component.14 We assessed this model with the AIC (Akaike Information Criterion). The non-cholera acute gastroenteritis-predicted denominator was then used with the known rotavirus-positive children to calculate alternative percent rotavirus positivity and reductions in rotavirus positivity. In addition, we analyzed trends only among the rotavirus-positive cases. We fit a negative binomial model to the pre-vaccine and the initial post-vaccine introduction rotavirus-positive hospitalization time series data (May 2013–December 2014) to predict the expected monthly number of rotavirus diarrhea hospital admissions in the absence of the rotavirus vaccination program from January 2015 to April 2019. Because coverage was low among children younger than 1 year in 2014, we included the entire year of 2014 as part of the pre-vaccine period to increase the pre-vaccine months for the model. We adjusted for seasonality by including calendar month, and secular trends by including year of admission. We assessed model fit with the Pearson χ2 statistic. We then plotted the expected number of diarrhea hospital admissions resulting from rotavirus against the recorded number in the vaccine era (January 2015–April 2019). To calculate the reduction in the absolute number of cases hospitalized as a result of rotavirus compared with the expected number, we included an indicator variable in the model for before and after rotavirus vaccine introduction and exponentiated the β of the indicator variable and its associated Wald 95% CI.
Data were organized using Microsoft Access (Microsoft Corp., Redmond, WA) and DHIS2, and analyzed the data using SAS (v.9.4; SAS Institute, Cary, NC). Figures were generated in Microsoft Excel.
Sentinel surveillance conducted through PRESEpi was considered routine public health surveillance and received a non-research determination from the CDC, Atlanta, GA. The Haiti Ethical Review Committee, Port-au-Prince, Haiti, also considered this activity to be non-research.
RESULTS
From May 2013 to April 2019, 4,353 children younger than 5 years of age were enrolled through the PRESEpi surveillance system at the two continuously reporting sites: Hopital St. Camille and Hopital St. Nicholas (Figure 1). Of these children, 2,642 (61%) were enrolled for diarrheal syndromes; of them, 2,268 (86%) had a rotavirus test result and were included in our analysis.
Figure 1.
EIA = enzyme immunoassay; PRESEpi = Project de la Renforcement de la Surveillance Epidémiologique (International Partners Established an Active, Integrated, Laboratory-Enhanced Sentinel Surveillance System).
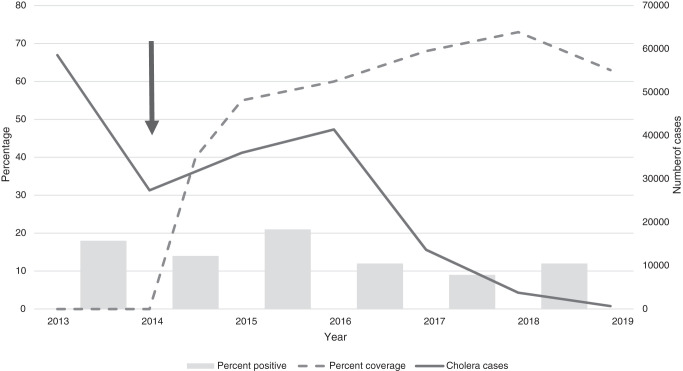
During the pre-vaccine surveillance period, 129 of 735 (19%) specimens tested were positive for rotavirus (Table 1). By 2015 to 2016, the second year after rotavirus vaccine introduction, the number of specimens tested annually had been reduced by more than 50% to 357, and was halved again to 137 specimens by 2018 to 2019, the fifth year after rotavirus vaccine introduction. The overall percentage of positive specimens declined by 22% the first year after introduction (2014–2015), increased by 17% in 2015 to 2016, and declined by 33%, 50%, and 33% in 2016 to 2017, 2017 to 2018, and 2018 to 2019, respectively. The absolute number of positive specimens declined each year to 16 rotavirus positive specimens in 2018 to 2019—an 88% reduction compared with 2013 to 2014. Figure 2 shows the annual percentage of specimens that tested positive for rotavirus among children younger than 60 months in our sample, plotted with the national percentage of rotavirus coverage among children younger than 12 months and the national absolute number of cholera cases of any age.
Table 1.
Number and percentage of rotavirus-positive specimens by age group and May to April surveillance year. Ouest and Artibonite departments, Haiti, 2013 to 2019
| Age group and surveillance year | No. tested | No. of rotavirus-positive specimens | Percentage of rotavirus-positive specimens | ||
|---|---|---|---|---|---|
| n | Decline (%) | % | Decline (%) | ||
| < 12 mo | |||||
| 2013–14 | 400 | 64 | Ref. | 16 | Ref. |
| 2014–15 | 231 | 28 | 56 | 12 | 25 |
| 2015–16 | 156 | 32 | 50 | 21 | –31 |
| 2016–17 | 147 | 19 | 70 | 13 | 19 |
| 2017–18 | 137 | 18 | 72 | 13 | 19 |
| 2018–19 | 64 | 6 | 91 | 9 | 44 |
| 12–59 mo | |||||
| 2013–14 | 334 | 65 | Ref. | 19 | Ref. |
| 2014–15 | 216 | 35 | 46 | 16 | 16 |
| 2015–16 | 201 | 42 | 35 | 21 | –11 |
| 2016–17 | 180 | 19 | 71 | 11 | 42 |
| 2017–18 | 129 | 7 | 89 | 5 | 74 |
| 2018–19 | 73 | 10 | 85 | 14 | 26 |
| < 60 mo | |||||
| 2013–14 | 734 | 129 | Ref. | 18 | Ref. |
| 2014–15 | 447 | 63 | 51 | 14 | 22 |
| 2015–16 | 357 | 74 | 43 | 21 | –17 |
| 2016–17 | 327 | 38 | 71 | 12 | 33 |
| 2017–18 | 266 | 25 | 81 | 9 | 50 |
| 2018–19 | 137 | 16 | 88 | 12 | 33 |
Figure 2.
Percentage of rotavirus-positive specimens among children younger than 59 months, national rotavirus vaccination coverage, and the national absolute number of suspected cholera cases in the Ouest and Artibonite Departments, Haiti, 2013 to 2019. The arrow indicates the year of rotavirus vaccine introduction.
Annual trends among infants younger than 12 months and children 12 to 59 months followed a similar pattern. The percentage of rotavirus-positive specimens was 16% among infants during the pre-vaccine period and declined by 44% to 9% in 2018 to 2019. The absolute number of positive specimens in infants was reduced by 91%, from 64 in 2013 to 2014 to six in 2018 to 2019. The percentage of rotavirus-positive specimens was 19% among children 12 to 59 months old during the pre-vaccine period and declined by 26% to 14% in 2018 to 2019. The absolute number of positive specimens in this age group was reduced by 91%, from 65 in 2013 to 2014 to 10 in 2018 to 2019.
When looking at the percentage of specimens that were positive for rotavirus by month, there is a clear seasonal pattern before and after rotavirus vaccine introduction, with the greatest rotavirus activity in December through April each year (Figure 3). There also appears to be a biennial pattern, with high, sharp peaks during the surveillance years, peaking in an even year, and flatter, longer periods of increased rotavirus activity in the surveillance years, peaking in odd years. For example, the months with the greatest percentage of positive specimens were February 2014 at 60%, January 2016 at 63%, and March 2018 at 47%. In the seasons peaking in odd years, the months with greatest rotavirus activity were April 2015, with 36% of specimens testing positive; January 2017, with 28% of specimens testing positive; and March 2019, with 30% of specimens testing positive.
Figure 3.
Number and percent of specimens positive for rotavirus per month at two hospitals in the Ouest and Artibonite Departments, Haiti, 2013 to 2019. The arrow indicates the month of rotavirus vaccine introduction. This figure appears in color at www.ajtmh.org.
The finite-mixture model predicted 82% of rotavirus-negative children included in this analysis in the pre-vaccine period and a median of 40% of children in the post-vaccine years could be attributed to cholera (Figure 4). Recalculating the percentage of positive specimens for rotavirus among children younger than 60 months, and excluding those predicted to be part of the cholera component, 42% were positive in 2013 to 2014, 18% in 2014 to 2015, 26% in 2015 to 2016, 16% in 2016 to 2017, 15% in 2017 to 2018, and 17% in 2018 to 2019. This represents a 38% reduction in rotavirus positivity in 2015 to 2016 and a 60% reduction in 2018 to 2019.
Figure 4.
Annual predicted number of rotavirus (Rota)-negative and cholera-positive specimens using a finite-mixture model and recalculated percent rotavirus positivity among cases of acute gastroenteritis from children younger than 5 years old in the Ouest and Artibonite Departments, Haiti, 2013 to 2019. The arrow indicates when the rotavirus vaccine was introduced.
The expected and the observed number of rotavirus-positive tests by month is presented in Figure 5. Because we included some months after vaccine introduction in the pre-vaccine period to ensure adequate data for our model, the expected number of rotavirus cases decreases annually, which indicates a year-over-year decrease in the absolute number of rotavirus-positive cases during the second half of 2014. In all post-rotavirus vaccine introduction years, the number of cases during the month of peak rotavirus activity predicted by the model projected is greater than the greatest number of observed cases during any single month. Controlling for admission month and year, there was a 21% (95% CI, –47 to 57) reduction in the observed number of rotavirus-positive specimens compared with the expected number from our negative binomial model among children younger than 1 year. Among children younger than 5 years, a 13% increase (–13; 95% CI, –83 to 30) occurred in the observed number of rotavirus-positive specimens compared with the expected number. During the early surveillance years after introduction (through April 2016), there was a 36% (95% CI, –11 to 63) reduction compared with data through April 2014.
Figure 5.
Monthly observed and expected number of rotavirus-positive specimens from children younger than 5 years old using a negative binomial model in the Ouest and Artibonite Departments, Haiti, 2013–2019. The arrow indicates when the rotavirus vaccine was introduced. Model fit: P = 0.014.
DISCUSSION
Following rotavirus vaccine introduction in Haiti in 2014, we found a decline of 22% to 50% in the percentage of rotavirus-positive specimens among children younger than 5 years old during the next 5 years, although there was a relative increase in the second year after rotavirus vaccine introduction. Overall, the percentage of specimens positive for rotavirus in our study in Haiti was less in the pre- and post-vaccine eras than has been reported in other countries.6 For example, on the east side of Hispaniola in the Dominican Republic, the proportion of diarrhea hospitalizations caused by rotavirus was estimated to be 62% during the pre-vaccine era, and the estimated proportion during the pre-vaccine ere from the Americas ranges from 30% to 38%.18,19 The influence of the cholera outbreak is one possible explanation for the discrepancy in relative rotavirus burden. In the earlier analysis of rotavirus using data from PRESEpi, the authors hypothesized the proportion of cases of acute gastroenteritis was a result of rotavirus diluted by the number of cholera cases; the cholera outbreak inflated the all-cause acute gastroenteritis denominator, and thus the percent positive of rotavirus was less than expected.12 Results from our finite-mixture model indicate that nearly half of rotavirus-negative children may be attributed to cholera, and rotavirus positivity during the pre-vaccine period may have been approximately 40% in the absence of a cholera outbreak. The impact of cholera on other diarrhea surveillance continues to be a factor and likely explains the relatively lower ecological impact of rotavirus vaccine introduction in Haiti compared with that observed by other countries in the region. This seems to be especially true in the early years after rotavirus vaccine introduction, when there were still many cases of cholera annually and the epidemiology was evolving rapidly.8,14 Taken together, our findings demonstrate substantial reductions in rotavirus hospitalizations among infants and young children in Haiti, and appear to be sustained during the past 3 surveillance years.
We observed a biennial pattern, in which seasons peaking in even years had higher, sharper peaks than seasons peaking in odd years. In the United States, a biennial rotavirus outbreak pattern has emerged since rotavirus vaccine introduction and is hypothesized to be caused by suboptimal vaccination coverage resulting from the accumulation of a cohort of children who are not protected against rotavirus disease by vaccination or natural infection. 20 To the best of our knowledge, this is the first example of a biennial trend in rotavirus activity documented outside the United States; although, interestingly, the high years in the United States (odd years) are the opposite of the high years observed in the PRESEpi data (even years). 21 Because we only have 1 year of pre-rotavirus vaccine testing data, we are unable to determine whether this pattern began before rotavirus vaccine introduction. However, this finding is consistent with lower vaccination coverage reported in Haiti.15 In addition to continuing to improve rotavirus vaccine coverage, ongoing surveillance for rotavirus should continue to monitor monthly rotavirus activity in Haiti.
In our analysis, the proportion of rotavirus-positive specimens increased during the second year after rotavirus vaccine introduction because the number of rotavirus-negative cases decreased faster than the rotavirus-positive cases. Expanding on conclusions from the previous pre-rotavirus vaccine PRESEpi analysis,12 we hypothesize that the decline in rotavirus-negative specimens in our analysis can be attributed in part to changes in care-seeking behavior and referral patterns in response to the cholera epidemic. In addition to anecdotal reports, there is some evidence for these unmeasured factors more broadly in Haiti.8,14,22–24 For example, when comparing demographic and health surveys at three time points (before the cholera outbreak in 2005–2006 during the cholera outbreak, before rotavirus vaccine introduction in 2012, and after rotavirus vaccine introduction in 2016–2017), the percentage of children younger than 5 years old with diarrhea in the previous 2 weeks was similar, but the percentage for whom treatment was sought increased by more than 30% in 2012 and 2016 to 2017 compared with 2005 to 2006.22–24 Although other explanations are possible, these findings suggest that changes in access to care and care-seeking behavior for acute gastroenteritis may be related temporally to the cholera outbreak. These changes likely impacted our findings in 2015 to 2016 and may have had a more subtle effect in other surveillance years. In addition, we were unable to account for other changes after the cholera outbreak, such as availability of oral rehydration solution and community knowledge of water, sanitation, and hygiene measures.
Our analysis has several limitations. Only 1 year of data before rotavirus vaccine introduction was available, so our results should be interpreted with care. We used several months of data after rotavirus vaccine introduction in 2014 in the negative binomial model as pre-vaccine data; this made our model more conservative and probably underestimated the number of expected cases during the post-vaccine period, but this may have introduced other biases. One year of pre-introduction data also increased our model’s confidence limits with each year. Second, test results were unavailable for some specimens; however, the number was consistent across surveillance years and we do not believe this biased our findings. Third, because this is an integrated surveillance system, some children may have been enrolled for more than one syndrome. By including all children younger than 5 years enrolled for diarrhea or with a stool collected, we attempted to capture all cases intended for rotavirus enrollment, but may have included children enrolled for other syndromes. In addition, some children were tested for cholera, but most were only tested for rotavirus. Fourth, this analysis includes data from two hospitals in two large cities in Haiti; it may not be representative of other parts of the country. In addition, cholera incidence and rotavirus vaccination coverage are heterogeneous in Haiti, and thus our findings may not be generalizable outside of the Artibonite and Ouest Departments. In addition, we did not have accurate population estimates for each hospital over time to calculate rates. Last, the complex situation resulting from the cholera outbreak in Haiti complicates interpretation of these data. Although each indicator is imperfect by itself, the fact that both the absolute number rotavirus-positive specimens and the percentage of rotavirus-positive specimens declined supports that the rotavirus vaccine is having a real and substantial impact on rotavirus hospitalizations.
We found strong evidence that the rotavirus vaccine reduced the burden of severe rotavirus diarrhea in Haiti 5 years after rotavirus vaccine introduction. Direct measures of vaccine performance, such as vaccine effectiveness, may provide additional useful information to decision makers and stakeholders in Haiti. Last, identification of circulating rotavirus genotypes in Haiti and continued surveillance to monitor seasonal activity, including the biennial pattern, are warranted.
ACKNOWLEDGMENTS
We acknowledge the PRESEpi team and field staff, especially Magdaline Sam, Marc Covens Junior Jean-Baptiste, Chedelene Rivière, and the participating children and their families for their contributions to this work.
REFERENCES
- 1. Crawford SE. et al. , 2017. Rotavirus infection. Nat Rev Dis Primers 3: 17083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tate JE, Burton AH, Boschi-Pinto C, Parashar UD, 2016. World Health Organization–coordinated global rotavirus surveillance: global, regional, and national estimates of rotavirus Mortality in children < 5 years of age, 2000–2013. Clin Infect Dis 62 (Suppl 2): S96–S105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Anon., 2009. Rotavirus vaccines: an update. Wkly Epidemiol Rec 84: 533–540. [PubMed] [Google Scholar]
- 4. Paternina-Caicedo A. et al. , 2015. Effect of rotavirus vaccine on childhood diarrhea mortality in five Latin American countries. Vaccine 33: 3923–3928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. World Health Organization , 2020. Vaccine in National Immunization Programme Update. Available at: https://www.who.int/immunization/monitoring_surveillance/VaccineIntroStatus.pptx. Accessed July 25, 2021.
- 6.Burnett E, Parashar UD, Tate JE, 2020. Global impact of rotavirus vaccination on diarrhea hospitalizations and deaths among children < 5 years old: 2006–2019. J Infect Dis 222: 1731–1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tohme RA. et al. , 2017. Expansion of vaccination services and strengthening vaccine-preventable diseases surveillance in Haiti, 2010–2016. Am J Trop Med Hyg 97: 28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guillaume Y, Ternier R, Vissieres K, Casseus A, Chery MJ, Ivers LC, 2018. Responding to cholera in Haiti: implications for the national plan to eliminate cholera by 2022. J Infect Dis 218 (Suppl 3): S167–S170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Juin S. et al. , 2017. Strengthening national disease surveillance and response: Haiti, 2010–2015. Am J Trop Med Hyg 97: 12–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Derby KS. et al. , 2014. Hospitalizations and deaths caused by diarrhea in children five years old and younger at four hospitals in Haiti, 2010–2012. Am J Trop Med Hyg 90: 291–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vinekar K. et al. , 2015. Hospitalizations and deaths because of respiratory and diarrheal diseases among Haitian children under five years of age, 2011–2013. Pediatr Infect Dis J 34: e238–e243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Steenland MW. et al. , 2013. Laboratory-confirmed cholera and rotavirus among patients with acute diarrhea in four hospitals in Haiti, 2012–2013. Am J Trop Med Hyg 89: 641–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Siddique AK. et al. , 2011. Epidemiology of rotavirus and cholera in children aged less than five years in rural Bangladesh. J Health Popul Nutr 29: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Direction d’Epidemiologie, des Laboratoires et de la Recherche, 2019. Rapport du Reseau National de Surveillance Cholera. Available at: https://mspp.gouv.ht/site/downloads/Profil%20statistique%20Cholera%2051SE%202019.pdf. Accessed July 25, 2021.
- 15. World Health Organization , 2020. Haiti: WHO and UNICEF Coverage Estimates of Immunization Coverage: 2019 Revision. Available at: https://www.who.int/immunization/monitoring_surveillance/data/hti.pdf. Accessed July 21, 2021.
- 16. SAS Institute , 2015. SAS/State 14.1 User’s Guide. Cary, NC: SAS Institute.
- 17. McLachlan G, Peel D, 2000. Finite Mixture Models. New York, NY: Wiley. [Google Scholar]
- 18. Linhares AC. et al. , 2011. Burden and typing of rotavirus group A in Latin America and the Caribbean: systematic review and meta-analysis. Rev Med Virol 21: 89–109. [DOI] [PubMed] [Google Scholar]
- 19. Aliabadi N. et al. , 2019. Global impact of rotavirus vaccine introduction on rotavirus hospitalisations among children under 5 years of age, 2008–16: findings from the Global Rotavirus Surveillance Network. Lancet Glob Health 7: e893–e903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tate JE. et al. , 2013. Trends in national rotavirus activity before and after introduction of rotavirus vaccine into the national immunization program in the United States, 2000 to 2012. Pediatr Infect Dis J 32: 741–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hallowell BD, Parashar UD, Curns A, DeGroote NP, Tate JE, 2019. Trends in the laboratory detection of rotavirus before and after implementation of routine rotavirus vaccination: United States, 2000–2018. MMWR Morb Mortal Wkly Rep 68: 539–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cayemittes M, Busangu MF, Bizimana JdD, Barrère, Sévère B, 2013. Haïti Enquête Mortalité, Morbidité et Utilisation des Services 2012. Calverton, MD: Ministère de la Santé Publique et de la Population-MSPP/Haïti, l’Institut Haïtien de l’Enfance-IHE, and ICF International. [Google Scholar]
- 23. Cayemittes M, Placide MF, Mariko S, Barrère B, Sévère B, 2007. Haiti Enquête Mortalité, Morbidité et Utilisation des Services 2005–2006. Calverton, MD: Institut Haïtien de l’Enfance and Macro International. [Google Scholar]
- 24.Institut Haïtien de l’Enfance – IHE/Haiti and ICF, 2018. Haiti Enquête Mortalité, Morbidité et Utilisation des Services 2016–2017 – EMMUS-VI. Pétion-Ville/Haïti: IHE/Haiti, ICF. Available at: http://dhsprogram.com/pubs/pdf/FR326/FR326.pdf. [Google Scholar]