Abstract
Aim: To examine whether improvement in flow-mediated endothelium-dependent dilatation (FMD) of the brachial artery and brachial-ankle pulse wave velocity (baPWV) has an additive effect on achieving optimal goals of traditional risk factors to reduce cardiovascular risk in patients with coronary artery disease (CAD).
Methods: We assessed 323 patients with CAD and impaired vascular function, defined as an impaired FMD of the brachial artery (<5.5%) and increased baPWV (>1,440 cm/sec). After FMD and baPWV measurements at 24 weeks of optimal medical treatment (OMT), the study patients were followed up for <60 months or until a composite of cardiac death, nonfatal myocardial infarction (MI), unstable angina, or ischemic stroke occurs.
Results: During the median follow-up period of 35 months, cardiovascular events occurred in 72 patients. Multivariate Cox hazards analysis showed that patients with an improvement in FMD and baPWV had the lowest probability of future cardiovascular events. In addition, the improvement in FMD and baPWV had a significant incremental effect on the predictive value of the achievement of optimal goals for blood pressure (BP), low-density lipoprotein cholesterol (LDL-C), and hemoglobin A1c (HbA1c) using net reclassification improvement (NRI) and integrated discrimination improvement (IDI).
Conclusions: The improvement in FMD and baPWV had additive effects on risk reduction of the achievement of the optimal goals of traditional risk factors in patients with CAD. Thus, serial measurements of FMD and baPWV may be useful for identifying CAD patients at residual risk for adverse cardiovascular events following OMT.
Keywords: Flow-mediated dilation, Endothelial function, Arterial stiffness, Pulse wave velocity, Residual risk
See editorial vol. 28: 1123-1125
Introduction
The optimal medical treatment (OMT) of multiple cardiovascular risk factors, including treatment for dyslipidemia, hypertension, and diabetes mellitus, reduces future cardiovascular events in patients with coronary artery disease (CAD) 1 , 2) . However, residual risks remain after achieving the optimal therapeutic goals of these multiple risk factors 3) . Therefore, identifying patients at a high residual risk of secondary cardiovascular events, even after achieving the optimal therapeutic goals in patients with CAD, is important.
Recent studies suggest that vascular dysfunction is not merely a local vascular occurrence; instead, it exists at multiple sites in the systemic vascular bed 4 , 5) . Vascular dysfunction, such as endothelial dysfunction and increased arterial stiffness, influences the development of atherosclerosis and leads to cardiovascular events 6 - 9) . Relevant vascular function tests, including flow-mediated dilation (FMD) of the brachial artery to assess endothelial function and pulse wave velocity (PWV) and subsequent arterial stiffness, have also been shown to be useful in assessing the risk of future cardiovascular events 10 - 13) . We have previously demonstrated that measurements of FMD and baPWV independently and additionally contribute to the predictability of secondary prevention for coronary events in patients with CAD 14) .
Several clinical studies have shown that endothelial vasomotor dysfunction and increased arterial stiffness are reversible after modifying the atherosclerotic risk factor by pharmacological intervention and lifestyle modification 11 , 13) . However, whether the improvement of these vascular parameters has additive effects on the predictive value of achieving the optimal therapeutic goals of traditional risk factors remains unclear.
Aim
This study examined whether the improvement in FMD of the brachial artery and baPWV has an additive effect on the achievement of the optimal therapeutic goals of traditional risk factors to reduce cardiovascular risk in patients with CAD.
Methods
Study Patients
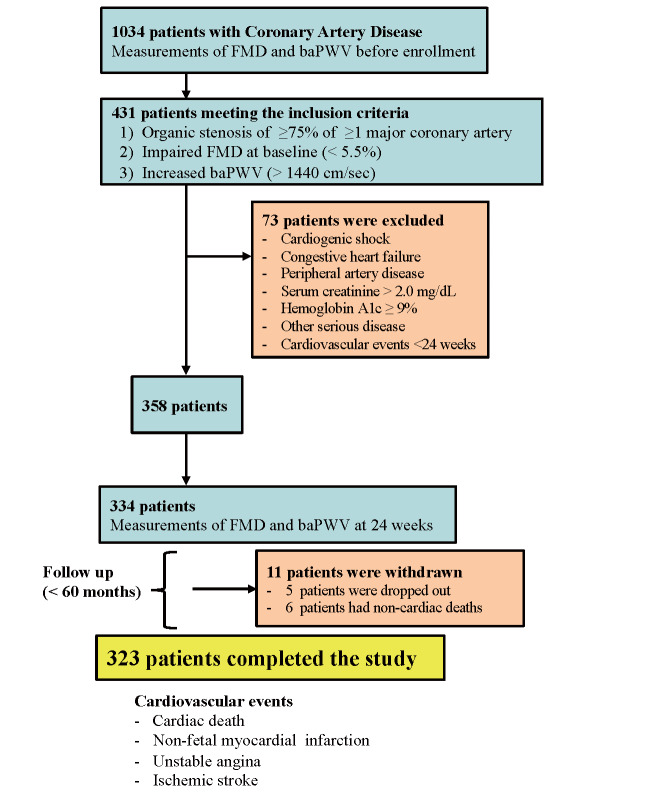
This study screened 1034 patients with CAD at the cardiology department of the University of Yamanashi Hospital between January 2005 and December 2012. All patients underwent routine noninvasive vascular measurements, including FMD of the brachial artery and baPWV before enrollment. The inclusion criteria were as follows: 1) angiographic documentation of an organic stenosis of ≥ 75% of ≥ 1 major coronary artery, 2) impaired FMD at baseline (<5.5%) according to our previous report 13) at the study enrollment, and 3) increased baPWV at baseline (>1,440 cm/sec; defined as the 50 th percentile of the distribution of the 1034 screened patients). The exclusion criteria were as follows: 1) cardiogenic shock; 2) congestive heart failure (New York Heart Association class III or IV); 3) peripheral artery disease; 4) major surgery and trauma, or severe infectious disease within 4 weeks prior to the study; 5) impaired renal function (serum creatinine of ≥ 2.0 mg/dL); 6) active liver disease; 7) uncontrolled diabetes mellitus [hemoglobin A1c (HbA1c) of ≥ 9%]; and 8) secondary hypertension. Overall, 431 patients who met the inclusion were enrolled ( Fig.1 ) . All patients provided written informed consent for participation at enrollment. This study was conducted following the guidelines approved by the Ethics Committee of the University of Yamanashi Hospital, and the investigation conformed to the principles outlined in the Declaration of Helsinki (1975).
Fig.1.
A flowchart of the enrollment of study patients
Study Protocol
After the baseline data, including FMD and baPWV, were obtained at our hospital, all study patients received individualized and optimized therapies, including medications and lifestyle changes, to reduce cardiovascular risk factors according to the guidelines 15) . Subsequently, binary measurements of FMD in the brachial artery and baPWV at 24 weeks after OMT were performed. In the present study, the optimal therapeutic targets for traditional risk factors were defined as follows: low-density lipoprotein cholesterol (LDL-C) of <100 mg/dL for lipid control, blood pressure (BP) of <140/90 mmHg (or <130/80 mmHg for patients with diabetes mellitus), and HbA1c of <7.0% for glycemic control.
After measurements of the FMD and baPWV at 24 weeks after enrollment, all study patients were followed up at a hospital by the patients’ primary physicians for a period of <60 months or until any of the following adverse cardiovascular events occurred: cardiac death, nonfatal myocardial infarction (MI), refractory unstable angina pectoris (uAP) requiring coronary revascularization, or ischemic stroke. The time to the first event was prospectively evaluated, and cardiac deaths were defined as sudden cardiac death or pump failure. Diagnosis of MI and uAP was confirmed by coronary angiography. Nonfatal MI was diagnosed by the presence of acute ischemic symptoms lasting ≥ 20 min within 48 h before hospital admission, electrocardiographic changes, and troponin T levels of >0.1 ng/mL. Follow-up data were obtained by the patients’ primary physicians every 3 months and then collected by the investigators (T. H., T. K., Y. S.) who were blinded to the patients’ characteristics at the time of enrollment. All endpoint data were strictly checked for accuracy, consistency, and completeness of follow-up by other investigators (T. Y., Y. W., K. N.), who also had no knowledge of the baseline clinical characteristics. Additional information was obtained from the physicians as required. Two of the investigators (T. N. and M. U.) checked all the data, carried out the analyses, and maintained the security of the data files.
Measurement of FMD of the Brachial Artery
Vasodilator responses in the brachial arteries were measured using B-mode ultrasound images with a 7.5-MHz linear array transducer (HP-5500, Phillips Corp., Tokyo, Japan) according to our previously validated method 13 , 14 , 16) . The brachial artery was scanned in the antecubital fossa in a longitudinal fashion. After baseline measurements of the diameter and flow velocity in the brachial artery, a BP cuff was placed around the forearm and inflated to a pressure of 250–300 mmHg for 5 min and then released. Diameter measurements during reactive hyperemia were taken 45–90 s after cuff deflation. Changes in vessel diameter in response to reactive hyperemia (FMD) were expressed as a percentage increase in diameter from the baseline value. Diameter responses were assessed at three locations along a 10-mm length of the artery, and the results were averaged for use in statistical analyses. All measurements were performed by the same experienced operators who were blinded to the patients’ baseline characteristics. In the present study, patients with an improvement in FMD were defined as (FMD at follow-up) minus (FMD at baseline) >0.
Measurement of baPWV
We measured baPWV by the volume plethysmographic method using an automatic waveform analyzer (VP-100; Colon Co. Ltd., Komaki, Japan) according to the manufacturer’s instructions and our previously validated method 10 , 11 , 14) . Before the measurement, patients were required to rest in the supine position for at least 5 min. Cuffs were wrapped around both upper arms and ankles. A phonogram, pulse volume waveform, BP, and heart rate were simultaneously recorded. The baPWV was calculated by measuring the time for the pulse wave to travel between the brachial and posterior tibial arteries. The mean values of the left and right baPWV were used in the statistical analyses. In the present study, patients with an improvement in baPWV were defined as (baPWV at 24 weeks) minus (baPWV at baseline) <0.
Statistical Analysis
All descriptive data are expressed as median and range (25 th and 75 th percentile) or frequencies (%). The median values and frequencies were compared between the two groups using the Mann–Whitney U test and Chi-square analysis, respectively. The Wilcoxon signed-rank tests were used to evaluate the changes in median values at baseline and follow-up. Kaplan–Meier survival analysis was performed in patients with improvement in FMD and baPWV, improvement in either FMD alone or baPWV alone, and without improvement in FMD and baPWV. The ability of the clinical parameters, achievements of optimal therapeutic goals for risk factors, and improvements in FMD and baPWV were assessed using univariate and multivariate Cox proportional hazards models. The univariate Cox hazards analyses included age, male sex, multivessel disease, creatinine, brain natriuretic peptide (BNP), LDL-C of <100 mg/dL, BP of <140/90 mmHg (<130/80 mmHg for patients with diabetes mellitus), HbA1c of <7%, and improvement in FMD and baPWV. To estimate the hazard ratio (HR) and 95% confidence interval (CI) of patients with improvement in either FMD or baPWV and patients with improvement in both FMD and baPWV, we used the patients without improvement in FMD and baPWV as the reference category. The predictive values of improvements in FMD and baPWV were assessed by multivariate Cox hazards analyses using three baseline statistical models of known risks and variables that were significant in univariate analysis. Model 1 consisted of age, male sex, creatinine, and achievement of three optimal therapeutic goals; model 2 consisted of age, male sex, multivessel disease, and achievement of three optimal therapeutic goals; and model 3 consisted of age, male sex, BNP, and achievement of three optimal therapeutic goals. Continuous variables were used at 1-SD increments in the Cox proportional hazards analysis, and dichotomous variables were coded 1 for the presence and 0 for the absence of the factor. We also assessed the net reclassification improvement (NRI) and integrated discrimination improvement (IDI) to analyze the predictive ability of the improvement in FMD and baPWV when they were added to the three baseline statistical models of known risks (models 1–3) 17 , 18) . All the presented probability values are two-tailed, with statistical significance inferred at p <0.05. Statistical analyses were carried out using STATA 13.0 (StataCorp, College Station, TX, USA).
Results
Comparisons of Baseline Traditional Risk Factors and Baseline Vascular Parameters between Patients with and without Cardiovascular Events
Overall, 334 patients who underwent binary measurements of FMD and baPWV were finally enrolled in the follow-up study ( Fig.1 ) . Five and six patients were withdrawn from the study as they could no longer be contacted and had noncardiac death (four due to malignancy, one due to respiratory disease, and one due to renal disease) during the follow-up, respectively. Finally, 323 patients completed the follow-up study [1–60 months; median, 35 months (interquartile range, 25–44)] ( Fig.1 ) . The baseline clinical characteristics of the 323 study patients are summarized in Table 1 . The study patients were classified into 94 patients with improvement in both FMD and baPWV, 73 patients with improvement in FMD alone, 61 patients with improvement in baPWV alone, and 95 patients without improvement in FMD and baPWV. Cardiovascular events occurred in 72 patients during the follow-up period (19 patients with cardiac death, 17 patients with nonfatal MI, 32 patients with uAP requiring coronary revascularization, and 4 patients with ischemic stroke). The baseline clinical characteristics of the study patients with or without cardiovascular events are summarized in Table 1 . Compared with patients without cardiovascular events, those with cardiovascular events had higher levels of age, creatinine, BNP, and HbA1c and higher frequency of diabetes mellitus and multivessel disease ( Table 1 ) .
Table 1. Comparisons of baseline clinical characteristics in patients with and without cardiovascular events.
| All patients ( n = 323) | With events ( n = 72) | Without events ( n = 251) | P -value | |
|---|---|---|---|---|
| Age (years) | 71 (69, 75) | 73 (70, 76) | 70 (69, 74) | <0.01 |
| Male sex, n (%) | 259 (80.2) | 61 (84.7) | 198 (78.9) | 0.23 |
| Smoking history, n (%) | 253 (78.3) | 59 (81.9) | 194 (77.3) | 0.40 |
| Diabetes mellitus, n (%) | 123 (38.1) | 39 (54.2) | 84 (33.5) | <0.01 |
| Dyslipidemia, n (%) | 216 (66.9) | 44 (61.1) | 172 (68.5) | 0.24 |
| Hypertension, n (%) | 244 (75.5) | 56 (77.8) | 188 (74.9) | 0.62 |
| Multivessel disease, n (%) | 129 (39.9) | 39 (54.2) | 90 (35.9) | 0.005 |
| Atrial fibrillation, n (%) | 16 (5.0) | 4 (5.6) | 12 (4.8) | 0.79 |
| Creatinine (mg/dL) | 1.1 (0.8, 1.3) | 1.2 (1.0, 1.5) | 1.1 (0.8, 1.3) | <0.01 |
| BNP (ng/mL) | 63 (41, 98) | 83 (49, 133) | 60 (41, 88) | 0.001 |
| HbA1c (%) | 5.7 (5.4, 6.5) | 6.3 (5.4) | 5.7 (5.3, 6.3) | 0.004 |
| LDL cholesterol (mg/dL) | 125 (109, 145) | 127 (107, 148) | 125 (109, 142) | 0.56 |
| Systolic BP (mmHg) | 154 (149, 158) | 153 (149, 158) | 155 (149, 158) | 0.57 |
| Diastolic BP (mmHg) | 78 (67, 87) | 77 (67, 88) | 78 (68, 86) | 0.66 |
| FMD (%) | 4.3 (3.5, 5.0) | 4.2 (3.5, 5.0) | 4.3 (3.5, 5.1) | 0.52 |
| baPWV (cm/sec) | 1722 (1623, 1868) | 1719 (1601, 1850) | 1723 (1628, 1878) | 0.31 |
| Medication use, n (%) | ||||
| Beta-blocker | 90 (27.9) | 15 (20.8) | 75 (29.9) | 0.13 |
| ACE-I/ARB | 160 (49.5) | 34 (47.2) | 126 (50.2) | 0.66 |
| Calcium antagonist | 58 (18.0) | 12 (16.7) | 46 (18.3) | 0.75 |
| Aspirin | 323 (100) | 72 (100) | 251 (100) | - |
| Statin | 311 (96.3) | 68 (94.4) | 243 (96.8) | 0.35 |
| Insulin | 11 (3.4) | 5 (5.9) | 6 (2.4) | 0.06 |
| Sulfonylurea | 29 (9.0) | 8 (11.1) | 21 (8.4) | 0.47 |
| Metformin | 17 (5.3) | 5 (6.9) | 12 (4.8) | 0.47 |
Data are expressed as median (25 th and 75 th percentiles) or number (%) of patients.
Diabetes mellitus was defined according to the American Diabetes Association criteria or use of antidiabetic medication. Dyslipidemia was defined by either fasting levels of LDL ≥ 140 mg/dL, triglycerides ≥ 150 mg/dL, or HDL ≤ 40 mg/dL, or use of cholesterol-lowering medications.
Hypertension was defined as > 140/90 mmHg or use of antihypertensive medication.
Abbreviations: BNP: Brain natriuretic peptide, FMD: Flosw mediated dilation, baPWV: Brachial-ankle pulse wave velocity, LDL: Low-density lipoprotein, ACE-I: Angiotensin-converting enzyme inhibitor, ARB: Angiotensin receptor blocker.
Comparisons of Atherosclerotic Risk Profiles and Use of Medications after 24 Weeks between Patients with and without Cardiovascular Events
The profiles of atherosclerotic risk factors and % achievements of optimal therapeutic targets for atherosclerotic risk factors after 24 weeks of medical treatment are summarized in Table 2 . In all study patients, the OMTs decreased the levels of LDL-C [125 (109, 145) mg/dL at baseline, 95 (84, 110) mg/dL at follow-up, p <0.001), systolic BP [154 (149, 158) mmHg at baseline, 126 (120, 140) mmHg at follow-up, p <0.001), diastolic BP [78 (67, 87) mmHg at baseline, 73 (66, 78) mmHg at follow-up, p <0.001), and HbA1c [5.9% (5.4%, 6.5%) at baseline, 5.8 (5.4, 6.8) at follow-up, p =0.04). Compared to patients with cardiovascular events, patients without cardiovascular events had lower levels of HbA1c, LDL-C, and systolic and diastolic BP at 24 weeks. No significant differences were found in the use of medications at 24 weeks. In addition, higher achievement of optimal goals for LDL-C, HbA1c, and BP and achievement of three optimal goals were observed in patients without cardiovascular events compared to those with cardiovascular events ( Table 2 ) .
Table 2. Comparisons of clinical characteristics at 24 weeks between patients with and without cardiovascular events.
| All patients ( n = 323) | With events ( n = 72) | Without events ( n = 251) | P -value | |
|---|---|---|---|---|
| HbA1c (%) | 5.7 (5.4, 6.8) | 6.3 (5.5, 7.1) | 5.6 (5.4, 6.5) | <0.01 |
| LDL cholesterol (mg/dL) | 96 (82, 113) | 103 (87, 115) | 95 (80, 112) | 0.04 |
| Systolic BP (mmHg) | 128 (120, 137) | 143 (140, 146) | 124 (120, 128) | <0.001 |
| Diastolic BP (mmHg) | 72 (66, 78) | 78 (66, 78) | 78 (71, 84) | <0.001 |
| Patients with LDL cholesterol <100 mg/dL, n (%) | 175 (54.2) | 30 (41.7) | 145 (57.8) | 0.02 |
| Patients with HbA1c <7%, n (%) | 243 (75.2) | 45 (62.5) | 198 (78.9) | 0.005 |
| Patients with BP <140/90 mmHg (<130/80 mmHg * ), n (%) | 200 (61.9) | 28 (38.9) | 172 (68.5) | <0.001 |
| Achievement of three optimal goals | 88 (27.2) | 10 (13.9) | 78 (31.1) | 0.004 |
| Medication use at 24 weeks, n (%) | ||||
| Beta-blocker | 120 (37.2) | 26 (36.1) | 94 (37.5) | 0.84 |
| ACE-I/ARB | 168 (52.0) | 38 (52.8) | 130 (51.8) | 0.88 |
| Calcium antagonist | 66 (20.4) | 13 (18.1) | 53 (21.1) | 0.57 |
| Aspirin | 323 (100) | 72 (100) | 251 (100) | - |
| Statin | 308 (95.4) | 67 (93.1) | 241 (96.0) | 0.29 |
| Insulin | 12 (3.7) | 2 (2.8) | 10 (4.0) | 0.63 |
| Sulfonylurea | 31 (9.6) | 9 (12.5) | 22 (8.8) | 0.34 |
| Metformin | 20 (6.2) | 7 (9.7) | 13 (5.2) | 0.16 |
| FMD (%) | 4.9 (3.1, 8.4) | 4.1 (2.7, 7.1) | 5.1 (3.2, 8.7) | 0.02 |
| baPWV (cm/sec) | 1752 (1630, 1891) | 1815 (1714, 1926) | 1737 (1611, 1876) | 0.003 |
| Patients with improvement in FMD, n (%) | 167 (51.7) | 25 (34.7) | 142 (56.6) | 0.001 |
| Patients with improvement in baPWV, n (%) | 164 (50.8) | 28 (38.9) | 136 (54.2) | 0.02 |
Abbreviations as in Table 1. * For patients with diabetes mellitus.
Comparisons of FMD and baPWV Values at 24 Weeks between Patients with and without Cardiovascular Events
The profiles of FMD and baPWV and % improvement in FMD and baPWV are summarized in Table 2 . Compared with patients with cardiovascular events, patients without cardiovascular events had higher levels of FMD and lower levels of baPWV at 24 weeks. Moreover, greater improvement in FMD and baPWV were observed in patients without cardiovascular events than in patients with cardiovascular events ( Table 2 ) .
Relationship between Improvement in FMD and baPWV and Atherosclerotic Risk Profiles
No significant differences were found in FMD and baPWV at baseline between patients who did and did not achieved three optimal therapeutic goals [FMD at baseline: 4.3% (3.4%, 5.2%) vs. 4.4% (3.7%, 5.1%), p =0.47; baPWV at baseline, 1709 (1608, 1832) cm/sec vs. 1725 (1623, 1876) cm/sec, p =0.38). However, FMD and baPWV at 24 weeks were more improved in patients who achieved three optimal therapeutic goals compared to those who did not (FMD at 24 weeks: 5.5% (3.6%, 8.8%) vs. 4.7% (3.1%, 8.1%), p =0.03; baPWV at 24 weeks: 1702 (1601, 1829) vs. 1762 (1649, 1899) cm/sec, p =0.01). Univariate logistic analyses showed that LDL-C of <100 mg/dL and BP of <140/90 mmHg (<130/80 mmHg for patients with diabetes mellitus) at 24 weeks were significantly associated with improvement in FMD and baPWV ( Table 3 ) . Moreover, when the reference group used was the patients who did not achieved three optimal goals, those who achieved three optimal goals at 24 weeks were significantly associated with improvement in both FMD and baPWV [odds ratio (OR), 6.81; 95% CI, 1.50–31.0, p =0.01, Table 3 ].
Table 3. Univariate logistic analysis for the relationship between improvement in both FMD and baPWV and clinical factors.
| Univariate analysis | P -value | ||
|---|---|---|---|
| OR | 95% CI | ||
| Age (yrs) | 1.00 | 0.96–1.04 | 0.95 |
| Sex, male | 1.43 | 0.76–2.71 | 0.27 |
| Multivessel disease | 1.02 | 0.63–1.68 | 0.91 |
| Creatinine (mg/dL) | 0.60 | 0.31–1.17 | 0.17 |
| BNP (pg/mL) | 1.02 | 0.80–1.29 | 0.90 |
| LDL cholesterol <100 mg/dL at 24 weeks | 3.28 | 1.92–5.61 | <0.01 |
| HbA1c <7% at 24 weeks | 0.90 | 0.48–1.34 | 0.37 |
| BP <140/90 mmHg (<130/80 mmHg * ), at 24 weeks | 1.89 | 1.10–3.25 | 0.02 |
| Number of achievements of three optimal goals | |||
| None (Ref) | 1.0 | ||
| 1 | 3.17 | 0.68–14.6 | 0.14 |
| 2 | 3.34 | 0.74–15.2 | 0.12 |
| 3 | 6.81 | 1.50–31.0 | 0.01 |
OR: odds ratio, CI: Confidence interval, other abbreviations as in Table 1.
The HR and 95% CI for BNP were estimated by a 1-SD increase in the variable.
* For patients with diabetes mellitus.
Predictive Value of Improvement in FMD and baPWV in the Prospective Study
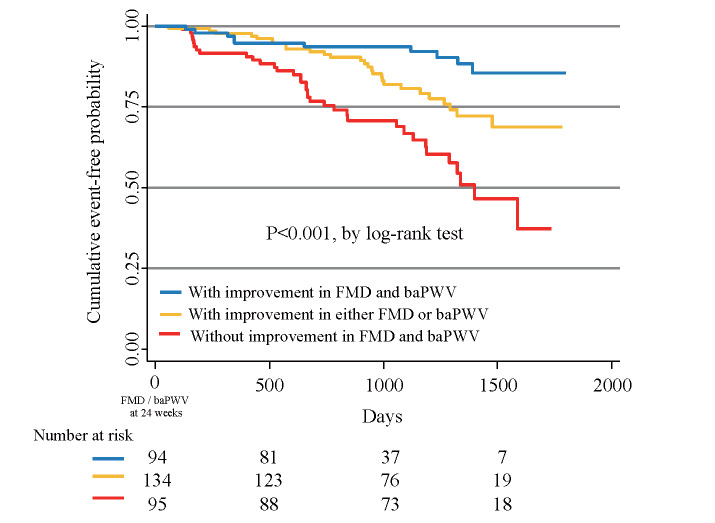
When the clinical outcomes were stratified according to the improvement in FMD and baPWV, either in combination or alone, Kaplan–Meier analysis showed that patients with improvement in both FMD and baPWV had the lowest probability of future cardiovascular events, followed by those with improvement in either FMD alone or baPWV alone and those without improvement in FMD and baPWV ( Fig.2 ) . As shown in Table 4 , univariate and multivariate Cox proportional hazards analyses showed that increasing age, creatinine, BNP, and the presence of multivessel disease significantly increased the risk of cardiovascular events. In addition, achievement of three optimal therapeutic goals for LDL-C, HbA1c, and BP significantly reduced the risk of cardiovascular events. When patients without improvement in FMD and baPWV were used as the reference group, patients with improvement in either FMD or baPWV demonstrated significantly reduced risk for cardiovascular events in univariate and multivariate Cox proportional hazards analyses (models 1 and 2). Patients with improvement in FMD and baPWV demonstrated significantly reduced risk for cardiovascular events in univariate and multivariate Cox proportional hazards analyses ( Table 4 ) .
Fig.2.
Kaplan–Meier curves showing event-free probability of cardiovascular events according to the improvement in FMD and baPWV
Table 4. Univariate and multivariate Cox proportional hazard analysis of risk factors for adverse clinical outcomes.
| Univariate analysis | Multivariate analysis | |||||||
|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | ||||||
| HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | |
| Age (yrs) | 1.09 | 1.03–1.14 ** | 1.08 | 1.03–1.14 *** | 1.10 | 1.04–1.16 ** | 1.09 | 1.04–1.15 ** |
| Sex, male | 1.44 | 0.89–3.29 | 1.71 | 0.89–3.29 | 1.61 | 0.84–3.09 | 1.80 | 0.94–3.45 |
| Creatinine (mg/dL) | 2.88 | 1.70–4.90 ** | 2.94 | 1.66–5.22 *** | − | − | ||
| Multivessel disease | 1.76 | 1.11–2.80 * | − | 1.76 | 1.10–2.81 * | − | − | |
| BNP (pg/mL) | 1.29 | 1.08–1.53 ** | − | − | 1.27 | 1.05–1.53 * | ||
| Achievement of three optimal goals | 0.19 | 0.08-0.48 *** | 0.25 | 0.10–0.62 ** | 0.28 | 0.11–0.70 ** | 0.25 | 0.10–0.64 ** |
| Improvement in FMD and baPWV | ||||||||
| None (Ref) | 1.0 | 1.0 | 1.0 | 1.0 | ||||
| Either FMD or baPWV | 0.45 | 0.27–0.75 ** | 0.54 | 0.32–0.90 * | 0.56 | 0.34–0.94 * | 0.64 | 0.38–1.08 |
| Both FMD and baPWV | 0.19 | 0.10–0.39 ** | 0.25 | 0.12–0.51 *** | 0.23 | 0.11–0.48 *** | 0.26 | 0.12–0.53 *** |
HR: Hazard ratio, other abbreviations as in Table 1. * p <0.05, ** p <0.01, *** p <0.001. The HR and 95% CI for BNP were estimated by a 1-SD increase in the variable.
Moreover, among the patients who achieved three optimal therapeutic goals ( n =88), using patients without improvement of FMD and baPWV as the reference group, patients with improvement in either FMD or baPWV alone tended to reduce the risk for cardiovascular events (HR, 0.31; 95% CI, 0.10–1.08; p =0.07), and patients with improvement in both FMD and baPWV had significantly reduced risks of cardiovascular events (HR, 0.14; 95% CI, 0.03–0.55; p <0.01). In addition, among the patients who did not achieved three optimal therapeutic goals ( n =229), patients with improvement in either FMD or baPWV and those with improvement in both FMD and baPWV demonstrated significantly reduced risks for cardiovascular events (improvement in either FMD or baPWV: HR, 0.50; 95% CI, 0.29–0.86; p <0.05; improvement in both FMD and baPWV: HR, 0.24; 95% CI, 0.11–0.55; p <0.01).
Additive Effects of Improvement in FMD and baPWV on the Predictive Values of the Achievement of Optimal Medical Goals
Category-free NRI and IDI were evaluated when the improvement in FMD and baPWV was added to the baseline models, including the achievement of three optimal therapeutic goals for LDL-C, HbA1c, and BP. The addition of the improvement in FMD and baPWV to the three baseline models of variables that were significant in the univariate Cox hazards analysis significantly improved category-free NRI and IDI ( Table 5 ) . Moreover, category-free NRI showed that 62.5% (model 1), 67% (model 2), and 61.1% (model 3) of patients with cardiovascular events had increased probability after adding the improvement in FMD and baPWV to the baseline model. Moreover, 58.2% (model 1), 62.2% (model 2), and 61.4% (model 3) of patients without cardiovascular events had decreased probability after adding the improvement in FMD and baPWV to the baseline model.
Table 5. Incremental effects of the improvement in both FMD and baPWV on the predictive value of the achievement of tradi- tional risk factors goals.
| Category-free NRI | IDI | |||
|---|---|---|---|---|
| Index | P-value | Index | P-value | |
| Baseline model 1: | ||||
| Age, sex, creatinine, achievement of three optimal goals | ||||
| Baseline model 1+improvement in both FMD and baPWV | 0.44 | <0.01 | 0.03 | <0.01 |
| Baseline model 2: | ||||
| Age, sex, multivessel disease, achievement of three optimal goals | ||||
| Baseline model 2+improvement in both FMD and baPWV | 0.43 | 0.001 | 0.03 | 0.004 |
| Baseline model 3: | ||||
| Age, sex, BNP, achievement of three optimal goals | ||||
| Baseline model 3+improvement in both FMD and baPWV | 0.47 | <0.001 | 0.04 | <0.001 |
NRI: net reclassification improvement, IDI: integrated discrimination improvement, other abbreviations as in Table 1.
Discussion
The present study demonstrated that the improvement in FMD and baPWV significantly reduced the risk of cardiovascular events in patients with CAD. Moreover, the addition of the improvement in FMD and baPWV to the achievement of therapeutic goals of risk factors after OMT significantly improved the risk models for adverse cardiovascular events in patients with CAD. Thus, the present findings indicate that serial measurement of both FMD and baPWV may be useful for identifying CAD patients at residual risk for adverse cardiovascular events after achieving optimal therapeutic goals.
PWV reflects arterial stiffening that is increased by adverse structural and functional alterations in the vascular wall 9 - 11) . These structural and functional abnormalities include endothelial dysfunction, medial hypertrophy, and elevated smooth muscle tone, which are associated with the development of atherosclerosis in the vascular tree 9 - 11) . Measuring baPWV, a parameter closely correlated with aortic PWV and carotid-femoral PWV, which are well-established vascular tests for the evaluation of atherosclerosis and cardiovascular risks 10 , 11) , is easy to perform. In previous studies, cardiovascular risk reduction has been shown to be related to the improvement of baPWV after modification of atherosclerotic risk burden, including BP lowering, LDL lowering, and glucose lowering 19 - 21) . FMD, a marker of endothelium-dependent dilation of the brachial artery, reflects systemic endothelial function, as well as atherosclerotic risk burden at the time of FMD measurement. We and others have shown that FMD values can be changed by modifying atherosclerotic risk factors 22 - 27) . Therefore, OMT for atherosclerotic risk may reduce the atherosclerotic burden in parallel with the improvement in both FMD and baPWV, suggesting that baseline assessment of FMD and baPWV may not necessarily reflect later status of atherosclerosis, especially in the patients newly treated for CAD. Thus, the predictive values of FMD and baPWV for future cardiovascular events may be considerably influenced by the treatment for atherosclerotic risks after baseline measurement. Accordingly, the present study showed that the improvement in FMD and baPWV was significantly associated with the achievement of optimal therapeutic goals for atherosclerotic risks, leading to reduction of cardiovascular events. Moreover, the selection of patients with impaired FMD and increased baPWV at baseline may partly account for the negative predictive value of baseline FMD and baPWV in the present study. These may explain our finding that the improvement in FMD and baPWV, but not FMD and baPWV at baseline, significantly reduces future cardiovascular events after achieving the therapeutic goals of OMT. Moreover, the present study showed that the predictability of the improvement of both FMD and baPWV for cardiovascular events was significant even in patients who did not achieved optimal therapeutic goals. Therefore, it might be possible that the improvement of vascular parameters not determined solely by the individual risk factor modification may be regarded as an index of all anti-atherosclerotic effects, including pleiotropic effects of medications, undetermined risk factor modification, and undetermined genetic disposition. Thus, the improvement of FMD and baPWV, a comprehensive analysis of atherosclerotic burden, may be predictive even in patients who did not achieve optimal therapeutic goals.
Recent studies have shown that several vascular parameters have similar ability to predict cardiovascular events but with increase predictive value when they are combined. We have shown that the combined addition of FMD and baPWV to the traditional risk factors significantly increases the predictability of secondary coronary events in patients with CAD. However, whether the changes in vascular parameters after OMT influence the prognostic values in patients with CAD remains unknown. In this sense, the present study clearly showed the utility of serial measurements of both FMD and baPWV to identify patients with CAD at a high residual risk of cardiovascular events after the achievement of optimal therapeutic goals for atherosclerotic risk factors.
It still remains unknown whether improvement of FMD and baPWV could be significant even in patients with preserved FMD and baPWV at baseline. Therefore, we assessed patients with preserved FMD and baPWV at baseline during the study period. In the present study, 168 patients had preserved FMD (>5.5%) and preserved baPWV (<1,400 cm/sec) at baseline, of which 117 (69.6%) patients had available follow-up FMD and baPWV data. During the follow-up period, cardiovascular events occurred in 11 (9.4%) patients. The incidence of cardiovascular events in this group was lower than those included in the present study ( p <0.05). These results may suggest that preserved FMD and baPWV at baseline reflect patients with lower risk for secondary cardiovascular events. Moreover, in contrast to patients included in the present study, univariate Cox hazards analysis showed that the improvement of both FMD and baPWV at 24 weeks was not significantly associated with cardiovascular events in patients with preserved FMD and baPWV at baseline (HR, 0.56; 95% CI, 0.10–4.4, p =0.55). Therefore, the improvement of vascular parameters might be unlikely to mediate relationship between cardiovascular events and OMT in patients with preserved FMD and baPWV at baseline. However, it still remains unclear that there is a discrepant finding regarding the predictive value of the improvement of FMD and baPWV among patients at different levels of vascular parameters at baseline. Therefore, larger clinical trials are needed to assess the relationship between the improvement of FMD and baPWV and clinical outcomes in broader spectrum of patients with CAD.
Study Limitations
This study had several limitations. First, the present study included a relatively small number of patients with CAD and impaired vascular function, which reduced the statistical power of the study. Second, we did not examine changes in FMD and baPWV after the second test of FMD and baPWV. Thus, whether favorable changes in FMD and baPWV could be sustained after the 24-week follow-up remains unclear. Therefore, other larger clinical trials using several measurements of FMD and baPWV for a longer duration are needed to assess the precise role of the improvement in FMD and baPWV in the pathogenesis and prognosis of patients with CAD. Third, despite the majority of the study patients having LDL-C of <100 mg/dL after 24 weeks of medical therapy, most study patients had LDL-C levels between 70 and 100 mg/dL. Recent guidelines suggest an optimal LDL-C goal of <70 mg/dL for patients with CAD and a very high atherosclerotic risk profile. It remains unclear whether FMD and baPWV are useful for risk assessment in patients with CAD who have LDL-C of <70 mg/dL. Fourth, recent clinical data suggested that the cutoff values of FMD and baPWV for the risk assessment of cardiovascular disease should be 4% and 1,400 cm/sec, respectevily 28) . However, we did not use these cutoff values for the inclusion criteria and definition of the improvement in FMD and baPWV. The effects of the normalization of FMD and baPWV using these cutoff values on the predictive values in patients with CAD still remain unknown. Therefore, other clinical studies are needed to assess the precise role of the improvement in FMD and baPWV using recently developed cutoff values in the pathogenesis and prognosis of patients with CAD after the achievement of more aggressive therapeutic goals for atherosclerotic risk factors. Fifth, although the medication used at baseline and 24 weeks were similar in patients with and without cardiovascular events, the achievement of optimal goals for traditional atherosclerotic risk factors was lower in patients with cardiovascular events, suggesting the presence of responder and nonresponder to anti-atherosclerotic medication. Therefore, it is possible that the undetermined genetic predisposition may also exist in patients who did not respond to interventions to reduce atherosclerotic risk factors. However, all study patients did not receive optimal medication such as beta-blocker, ACE-I, ARB, and statin, during the study period, suggesting that the patients were not completely optimized with respect to medical treatment. Therefore, a larger, prospective trial using the same type of OMT needs to be performed to confirm whether FMD and baPWV measurements may predict future cardiovascular events after OMTs. Finally, this study included patients with atrial fibrillation that might affect baPWV and FMD values. Therefore, caution should be used when interpreting the prognostic values of FMD and baPWV for patients with atrial fibrillation.
Conclusions
The addition of the improvement in both FMD and baPWV to the achievement of optimal risk factor goals significantly reduced the risk of adverse cardiovascular events in patients with CAD. Thus, serial measurements of FMD and baPWV may be useful for identifying patients at residual risk for adverse cardiovascular events after OMTs for patients with CAD.
Acknowledgement
None.
Notice of Grant Support
This study was supported by grants-in-aid for Scientific Research (C-17K09491) from the Ministry of Education, Culture, Sports, Science, and Technology, Tokyo, Japan.
COI
K.K. received scholarship donations from Takeda, Daiichi Sankyo, Astellas, Boehringer Ingelheim, MSD, Boston Scientific Japan, Abbott, Medtronic, Biotronik Japan, and ST Jude Medical. The other authors declare no conflicts of interest.
References
- 1).Smith SC Jr, Allen J, Blair SN, Bonow RO, Brass LM, Fonarow GC, Grundy SM, Hiratzka L, Jones D, Krumholz HM, Mosca L, Pasternak RC, Pearson T, Pfeffer MA, Taubert KA; AHA/ACC; National Heart, Lung, and Blood Institute. AHA/ACC guidelines for secondary prevention for patients with coronary and other atherosclerotic vascular disease: 2006 update endorsed by the National Heart, Lung, and Blood Institute. J Am Coll Cardiol, 2006; 47: 2130-2139 [DOI] [PubMed] [Google Scholar]
- 2).Ford ES, Ajani UA, Croft JB, Critchley JA, Labarthe DR, Kottke TE, Giles WH, Capewell S. Explaining the decrease in U.S. deaths from coronary disease, 1980-2000. N Engl J Med, 2007; 356: 2388-2398 [DOI] [PubMed] [Google Scholar]
- 3).Kaasenbrood L, Boekholdt SM, van der Graaf Y, Ray KK, Peters RJ, Kastelein JJ, Amarenco P, LaRosa JC, Cramer MJ, Westerink J, Kappelle LJ, de Borst GJ, Visseren FL. Distribution of estimated 10-year risk of recurrent vascular events and residual risk in a secondary prevention population. Circulation, 2016; 134: 1419-1429 [DOI] [PubMed] [Google Scholar]
- 4).Goldstein JA, Demetriou D, Grines CL, Pica M, Shoukfeh M, O’Neill WW. Multiple complex coronary plaques in patients with acute myocardial infarction. N Engl J Med, 2000; 343: 915-922 [DOI] [PubMed] [Google Scholar]
- 5).Rothwell PM, Villagra R, Gibson R, Donders RC, Warlow CP. Evidence of a chronic systemic cause of instability of atherosclerotic plaques. Lancet, 2000; 355: 19-24 [DOI] [PubMed] [Google Scholar]
- 6).Widlansky ME, Gokce N, Keaney JF, Jr., Vita JA. The clinical implications of endothelial dysfunction. J Am Coll Cardiol, 2003; 42: 1149-1160 [DOI] [PubMed] [Google Scholar]
- 7).Bonetti PO, Lerman LO, Lerman A. Endothelial dysfunction: A marker of atherosclerotic risk. Arterioscler Thromb Vasc Biol, 2003; 23: 168-175 [DOI] [PubMed] [Google Scholar]
- 8).Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: A systematic review and meta-analysis. J Am Coll Cardiol, 2010; 55: 1318-1327 [DOI] [PubMed] [Google Scholar]
- 9).Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, Pannier B, Vlachopoulos C, Wilkinson I, Struijker-Boudier H; European Network for Non-invasive Investigation of Large Arteries. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J, 2006; 27: 2588-2605 [DOI] [PubMed] [Google Scholar]
- 10).Tomiyama H, Yamashina A. Non-invasive vascular function tests: Their pathophysiological background and clinical application. Circ J, 2010; 74: 24-33 [DOI] [PubMed] [Google Scholar]
- 11).Tomiyama H, Koji Y, Yambe M, Shiina K, Motobe K, Yamada J, Shido N, Tanaka N, Chikamori T, Yamashina A. Brachial - ankle pulse wave velocity is a simple and independent predictor of prognosis in patients with acute coronary syndrome. Circ J, 2005; 69: 815-822 [DOI] [PubMed] [Google Scholar]
- 12).Kitta Y, Nakamura T, Kodama Y, Takano H, Umetani K, Fujioka D, Saito Y, Kawabata K, Obata JE, Ichigi Y, Mende A, Kugiyama K. Endothelial vasomotor dysfunction in the brachial artery is associated with late in-stent coronary restenosis. J Am Coll Cardiol, 2005; 46: 648-655 [DOI] [PubMed] [Google Scholar]
- 13).Kitta Y, Obata JE, Nakamura T, Hirano M, Kodama Y, Fujioka D, Saito Y, Kawabata K, Sano K, Kobayashi T, Yano T, Nakamura K, Kugiyama K. Persistent impairment of endothelial vasomotor function has a negative impact on outcome in patients with coronary artery disease. J Am Coll Cardiol, 2009; 53: 323-330 [DOI] [PubMed] [Google Scholar]
- 14).Sugamata W, Nakamura T, Uematsu M, Kitta Y, Fujioka D, Saito Y, Kawabata K, Obata JE, Watanabe Y, Watanabe K, Kugiyama K. Combined assessment of flow-mediated dilation of the brachial artery and brachial-ankle pulse wave velocity improves the prediction of future coronary events in patients with chronic coronary artery disease. J Cardiol, 2014; 64: 179-184 [DOI] [PubMed] [Google Scholar]
- 15).Gibbons RJ, Abrams J, Chatterjee K, Daley J, Deedwania PC, Douglas JS, Ferguson TB Jr, Fihn SD, Fraker TD Jr, Gardin JM, O’Rourke RA, Pasternak RC, Williams SV, Gibbons RJ, Alpert JS, Antman EM, Hiratzka LF, Fuster V, Faxon DP, Gregoratos G, Jacobs AK, Smith SC Jr. ACC/AHA 2002 guideline update for the management of patients with chronic stable angina-summary article: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines (Committee on the Management of Patients With Chronic Stable Angina). J Am Coll Cardiol, 2003; 41: 159-168 [DOI] [PubMed] [Google Scholar]
- 16).Nakamura T, Kitta Y, Uematsu M, Sugamata W, Hirano M, Fujioka D, Sano K, Saito Y, Kawabata K, Obata JE, Kugiyama K. Ultrasound assessment of brachial endothelial vasomotor function in addition to carotid plaque echolucency for predicting cardiovascular events in patients with coronary artery disease. Int J Cardiol, 2013; 167: 555-560 [DOI] [PubMed] [Google Scholar]
- 17).Pencina MJ, D’Agostino RB, Sr., D’Agostino RB, Jr., Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med, 2008; 27: 157-172; discussion 207-212 [DOI] [PubMed] [Google Scholar]
- 18).Cook NR, Ridker PM. Advances in measuring the effect of individual predictors of cardiovascular risk: the role of reclassification measures. Ann Intern Med, 2009; 150: 795-802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19).Zheng M, Huo Y, Wang X, Xu X, Qin X, Tang G, Xing H, Fan F, Li J, Zhang Y, Wang B, Xu X, Yang X, Chen Y, Qian G. A prospective study on pulse wave velocity (PWV) and response to anti-hypertensive treatments: PWV determines BP control. Int J Cardiol, 2015; 178: 226-231 [DOI] [PubMed] [Google Scholar]
- 20).Jia X, Wei M, Fu X, Gu X, Fan W, Zhang J, Xue L. Intensive cholesterol-lowering therapy improves large artery elasticity in acute myocardial infarction patients. Heart Vessels, 2009; 24: 340-346 [DOI] [PubMed] [Google Scholar]
- 21).Tomiyama H, Miwa T, Kan K, Matsuhisa M, Kamiya H, Nanasato M, Kitano T, Sano H, Ohno J, Iida M, Sata M, Yamada H, Maemura K, Tanaka A, Murohara T, Node K. Impact of glycemic control with sitagliptin on the 2-year progression of arterial stiffness: A sub-analysis of the PROLOGUE study. Cardiovasc Diabetol, 2016; 15: 150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22).Nakamura T, Uematsu M, Yoshizaki T, Kobayashi T, Watanabe Y, Kugiyama K. Improvement of endothelial dysfunction is mediated through reduction of remnant lipoprotein after statin therapy in patients with coronary artery disease. J Cardiol, 2019 [DOI] [PubMed] [Google Scholar]
- 23).Nakamura T, Hirano M, Kitta Y, Fujioka D, Saito Y, Kawabata K, Obata JE, Watanabe Y, Watanabe K, Kugiyama K. A comparison of the efficacy of combined ezetimibe and statin therapy with doubling of statin dose in patients with remnant lipoproteinemia on previous statin therapy. J Cardiol, 2012; 60: 12-17 [DOI] [PubMed] [Google Scholar]
- 24).Nakamura T, Takano H, Umetani K, Kawabata K, Obata JE, Kitta Y, Kodama Y, Mende A, Ichigi Y, Fujioka D, Saito Y, Kugiyama K. Remnant lipoproteinemia is a risk factor for endothelial vasomotor dysfunction and coronary artery disease in metabolic syndrome. Atherosclerosis, 2005; 181: 321-327 [DOI] [PubMed] [Google Scholar]
- 25).Chen JD, Liu M, Chen XH, Yang ZJ. Effect of Angiotensin receptor blockers on flow-mediated vasodilation: A meta-analysis of randomized controlled trials. Cardiology, 2015; 131: 69-79 [DOI] [PubMed] [Google Scholar]
- 26).Jensterle M, Sebestjen M, Janez A, Prezelj J, Kocjan T, Keber I, Pfeifer M. Improvement of endothelial function with metformin and rosiglitazone treatment in women with polycystic ovary syndrome. Eur J Endocrinol, 2008; 159: 399-406 [DOI] [PubMed] [Google Scholar]
- 27).Papathanassiou K, Naka KK, Kazakos N, Kanioglou C, Makriyiannis D, Pappas K, Katsouras CS, Liveris K, Kolettis T, Tsatsoulis A, Michalis LK. Pioglitazone vs glimepiride: Differential effects on vascular endothelial function in patients with type 2 diabetes. Atherosclerosis, 2009; 205: 221-226 [DOI] [PubMed] [Google Scholar]
- 28).Tanaka A, Tomiyama H, Maruhashi T, Matsuzawa Y, Miyoshi T, Kabutoya T, Kario K, Sugiyama S, Munakata M, Ito H, Ueda S, Vlachopoulos C, Higashi Y, Inoue T, Node K; Physiological Diagnosis Criteria for Vascular Failure Committee. Physiological Diagnostic Criteria for Vascular Failure. Hypertension, 2018; 72: 1060-1071 [DOI] [PubMed] [Google Scholar]