Abstract
Using provisional or opportunistic data, three nationwide studies (The Netherlands, the USA and Denmark) have identified a reduction in preterm or extremely preterm births during periods of COVID-19 restrictions. However, none of the studies accounted for perinatal deaths. To determine whether the reduction in extremely preterm births, observed in Denmark during the COVID-19 lockdown, could be the result of an increase in perinatal deaths and to assess the impact of extended COVID-19 restrictions, we performed a nationwide Danish register-based prevalence proportion study. We examined all singleton pregnancies delivered in Denmark during the COVID-19 strict lockdown calendar periods (March 12–April 14, 2015-2020, N = 31,164 births) and the extended calendar periods of COVID-19 restrictions (February 27–September 30, 2015-2020, N = 214,862 births). The extremely preterm birth rate was reduced (OR 0.27, 95% CI 0.07 to 0.86) during the strict lockdown period in 2020, while perinatal mortality was not significantly different. During the extended period of restrictions in 2020, the extremely preterm birth rate was marginally reduced, and a significant reduction in the stillbirth rate (OR 0.69, 0.50 to 0.95) was observed. No changes in early neonatal mortality rates were found.
Conclusion: Stillbirth and extremely preterm birth rates were reduced in Denmark during the period of COVID-19 restrictions and lockdown, respectively, suggesting that aspects of these containment and control measures confer an element of protection. The present observational study does not allow for causal inference; however, the results support the design of studies to ascertain whether behavioural or social changes for pregnant women may improve pregnancy outcomes.
|
What is Known: • The aetiologies of preterm birth and stillbirth are multifaceted and linked to a wide range of socio-demographic, medical, obstetric, foetal, psychosocial and environmental factors. • The COVID-19 lockdown saw a reduction in extremely preterm births in Denmark and other high-income countries. An urgent question is whether this reduction can be explained by increased perinatal mortality. | |
|
What is New: • The reduction in extremely preterm births during the Danish COVID-19 lockdown was not a consequence of increased perinatal mortality, which remained unchanged during this period. • The stillbirth rate was reduced throughout the extended period of COVID-19 restrictions. |
Supplementary Information
The online version contains supplementary material available at 10.1007/s00431-021-04297-4.
Keywords (MeSH): Stillbirth; Perinatal death; COVID-19; Epidemics; Infant, premature; Infant, extremely premature
Introduction
COVID-19 containment and control policies were implemented worldwide in response to the SARS-Cov-2 pandemic. The preventative and protective measures placed on communities in order to reduce viral transmission created an opportunistic experiment [1] which may add to our future understanding of what causes preterm birth (PTB) [2]. A Danish nationwide study first described a dramatic reduction in extremely PTBs (xPTB) during the strict lockdown period [3]. Following this finding, similar reductions in PTBs were reported from the Netherlands [1], Japan [4], Italy [5], Tennessee [6], New York [7], Israel [8] and the USA [9], and a report from Ireland described a reduced proportion of very low birth weight babies [10]. However, smaller studies from California [11], Philadelphia [12], Israel [13], Spain [14] and London [15], as well as a recent nationwide Swedish study [16], could not confirm these findings.
A recent meta-analysis indicated that during the COVID-19 pandemic high-income countries generally saw a reduction in PTBs and stillbirths, while low- to middle-income countries (LMIC) saw increases [17]. Notably, there are very few reports from LMIC. The conflicting findings can in part be explained by health system inefficiencies and/or an inability to deal adequately with the pandemic [17]. In Nepal, for instance, the number of women giving birth in institutions dropped precipitously [18]. These regional differences are further supported by a publication reporting data from 17 countries [19], where the xPTB rate was reduced between 11 and 22% in the European region and increased by 48% in China and India. Furthermore, some studies have reported elevated stillbirth rates [5, 13, 15] and a study from Nepal reported an increase in both PTBs and stillbirths during their lockdown periods [18].
Stillbirths and early neonatal mortality rates were not assessed in the three nationwide studies from The Netherlands, the USA and Denmark [1, 3, 9]. Since PTB rates could be reduced in response to increasing perinatal mortality rates [20], there is a need to assess live birth rates alongside perinatal mortality rates (stillbirth and early neonatal death) during the different periods studied. Accordingly, this study aimed to use data pertaining to all Danish pregnancies and births, captured in the extensive nationwide electronic registers to study the prevalence of PTB, stillbirth and early neonatal mortality from singleton pregnancies during the first strict lockdown period in Denmark and during the continuous period of COVID-19 restrictions. Furthermore, we describe the stringency of Danish COVID-19 policies and the timeline of their implementation in order to contextualize our findings.
Materials and methods
Study design and participants
The study is a retrospective register-based, nationwide study comparing the prevalence’s of PTB and perinatal death (stillbirth and early neonatal death defined as death within the first 7 days of life) in the Danish COVID-19 lockdown period with the same calendar periods in the preceding 5 years. All singleton births, born with a gestational age ≥ 22 + 0 weeks, registered in these periods, were included and the outcome, i.e. stillbirth, live birth at specific gestational ages, as well as death within the early neonatal period (first seven days of life), was registered. Induced abortions were excluded.
Data sources
Participants were identified using the extensive population-based registers available in Denmark [21], specifically The Danish Civil Registration System [22], the Medical Birth Registry [23] and The Danish National Patient Registry (LPR) [24] (LPR2 and, from February 2019, LPR3). Country-specific information about stringency and temporality of COVID-19 lockdown measures was obtained from the Oxford COVID-19 Government Response Tracker [25]. Mobility information from cell phone registrations was obtained from Google COVID-19 Community Mobility Reports [26].
Study period and outcomes
Births taking place between March 12 and April 14, 2020, constituted the group exposed to strict lockdown measures, and births between February 27 and September 30, 2020, constituted the broader group exposed to COVID-19 restrictions. The unexposed, control group, was the aggregated deliveries in the calendar periods February 27–September 30 and March 12–April 14, 2015–2019, respectively.
Statistical analysis
Prevalences were compared using Fisher’s exact test and proportionality test. Data analysis was performed using R version 4.0.3.
Results
The Danish lockdown
A strict lockdown was in effect from March 12 to April 14, 2020, followed by varying COVID-19 restrictions from April 15 to the end of this study, September 30, 2020. The chronological development of the official Danish response to the COVID-19 pandemic is summarized in Fig. 1, and the instituted measures, as well as behavioural effects, are described in detail in the Appendix and Fig. 1A, 1B in the Supplement.
Fig. 1.
The timeline of COVID-19 events in Denmark leading up to the lockdown. Different categories of events (orienting the public = orientation, published guidelines = guidelines, local cases and local transmission = cases/transmission, policy response = policy response, the WHO declaration of a pandemic = WHO, the declaration of the lockdown = lockdown declaration) are indicated by different colours. Data summarized from [31, 54, 55]
Birth and mortality rates during the strict lockdown
A total of 31,164 pregnancies with gestational age ≥ 22 + 0 weeks were registered in Denmark during the period March 12–April 14, in 2015–2020. No differences in birth rates or perinatal mortality rates were found between the year 2020 and preceding years 2015–2019 (Table 1). However, the xPTB rate was significantly reduced (OR 0.27, 95% CI 0.07–0.86) while the other PTB groups were not changed (Table 2). There were no differences in gestational age-dependent stillbirth rates, perinatal mortality and neonatal mortality rates (Table 2).
Table 1.
Number of births, stillbirths and mortality rates in the lockdown (March 12–April 14) and extended period of COVID-19 restrictions (February 27–September 30)
| 2020 | 2015–2019 | 2020 vs 2015–2019 | |
|---|---|---|---|
| Strict lockdown (March 12–April 14) | |||
| Births | N | Mean (SD) | |
| Total births | 5013 | 5230.2 (207.4) | |
| Total live births | 4999 | 5215.0 (205.4) | |
| % male | 51.8 | 51.3 (0.62) | |
| Mortality and mortality rates | N (%) | Mean (SD) | OR (95% CI) |
| Perinatal mortality | 19 (0.38) | 23.2 (3.4) | 0.86 (0.44, 1.66) |
| Stillbirths | 14 (0.28) | 15.4 (6.1) | 0.97 (0.43, 2.17) |
| Early neonatal mortality (≤ 7 days) | 5 (0.10) | 7.8 (2.9) | 0.65 (0.17, 2.26) |
| Very early mortality (< 24 h) | 3 (0.10) | 5.6 (1.5) | 0.52 (0.08, 2.44) |
| Period of COVID-19 restrictions (February 27–September 30) | |||
| Births | N | Mean (SD) | |
| Total births | 35,394 | 35,893.6 (1053.9) | |
| Total live births | 35,326 | 35,793.4 (1048.3) | |
| % male | 51.4 | 51.5 (0.21) | |
| Mortality and mortality rates | N (%) | Mean (SD) | OR (95% CI) |
| Perinatal mortality | 111 (0.31) | 148.2 (25.8) | 0.76 (0.29, 0.98) |
| Stillbirths | 68 (0.19) | 100.2 (14.9) | 0.69 (0.50, 0.95) |
| Early neonatal mortality (≤ 7 days) | 43 (0.12) | 48.0 (11.8) | 0.91 (0.59, 1.40) |
| Very early mortality (< 24 h) | 22 (0.06) | 28.0 (8.7) | 0.80 (0.43, 1.44) |
Table 2.
Live births and stillbirths as a function of gestational age at birth in the lockdown (March 12–April 14) and extended period of COVID-19 restrictions (February 27–September 30)
| GA | 2020 | 2015–2019 | 2020 vs 2015–2019 | |
|---|---|---|---|---|
| Lockdown (March 12–April 14) | ||||
| Weeks + days | N (%) | Mean (SD) | OR (95% CI) | |
| Live births | ||||
| Extremely preterm | ≤ 27 + 6 | 4 (0.08) | 15.0 (3.2) | 0.27 (0.07, 0.86) |
| Very preterm | 28 + 0–31 + 6 | 18 (0.36) | 23.4 (1.6) | 0.80 (0.41, 1.56) |
| Moderate preterm | 32 + 0–36 + 6 | 202 (4.03) | 217.4 (7.3) | 0.96 (0.78, 1.17) |
| Term | 37 + 0–41 + 6 | 4731 (94.37) | 4835.0 (206.1) | 1.13 (0.95, 1.34) |
| Late term | ≥ 42 + 0 | 33 (0.66) | 40.6 (3.8) | 0.83 (0.51, 1.34) |
| Missing GA | 11 (0.22) | 84.0 (39.5) | ||
| Stillbirths | ||||
| Extremely preterm | ≤ 27 + 6 | 4 (0.08) | 6.2 (3.0) | 0.55 (0.08, 3.28) |
| Very preterm | 28 + 0–31 + 6 | 4 (0.08) | 2.6 (1.10) | 1.57 (0.21, 13.43) |
| Moderate preterm | 32 + 0–36 + 6 | 2 (0.04) | 2.4 (1.1) | 1.00 (0.06, 15.94) |
| Term | 37 + 0–41 + 6 | 4 (0.08) | 3.2 (1.5) | 1.45 (0.19, 12.44) |
| Late term | ≥ 42 + 0 | 0 (0.00) | 0.0 (0.0) | 0.00 (0.00, inf) |
| Missing GA | 0 (0.00) | 0.0 (0.0) | ||
| Period of COVID-19 restrictions (February 27–September 30) | ||||
| Weeks + days | N (%) | Mean (SD) | OR (95% CI) | |
| Live births | ||||
| Extremely preterm | ≤ 27 + 6 | 58 (0.16) | 74.6 (4.9) | 0.79 (0.55, 1.12) |
| Very preterm | 28 + 0–31 + 6 | 150 (0.43) | 165.8 (6.4) | 0.92 (0.73, 1.15) |
| Moderate preterm | 32 + 0–36 + 6 | 1410 (3.98) | 1468.6 (45.8) | 0.98 (0.90, 1.05) |
| Term | 37 + 0–41 + 6 | 33,338 (94.19) | 33,242 (903.4) | 1.38 (1.29, 1.46) |
| Late term | ≥ 42 + 0 | 241 (0.68) | 289.4 (23.4) | 0.85 (0.71, 1.01) |
| Missing GA | 129 (0.36) | 550.8 (249.0) | ||
| Stillbirths | ||||
| Extremely preterm | ≤ 27 + 6 | 22 (0.06) | 33.4 (6.4) | 0.97 (0.47, 1.97) |
| Very preterm | 28 + 0–31 + 6 | 9 (0.03) | 11.6 (3.6) | 1.13 (0.39, 3.13) |
| Moderate preterm | 32 + 0–36 + 6 | 16 (0.05) | 20.0 (6.3) | 1.23 (0.54, 2.76) |
| Term | 37 + 0–41 + 6 | 21 (0.06) | 32.4 (6.1) | 0.95 (0.46, 1.94) |
| Late term | ≥ 42 + 0 | 0 (0.00) | 0.2 (na) | 0.00 (0.00, inf) |
| Missing GA | 0 (0.00) | 2.6 (1.7) | ||
Birth and mortality rates during the extended period of COVID-19 restrictions
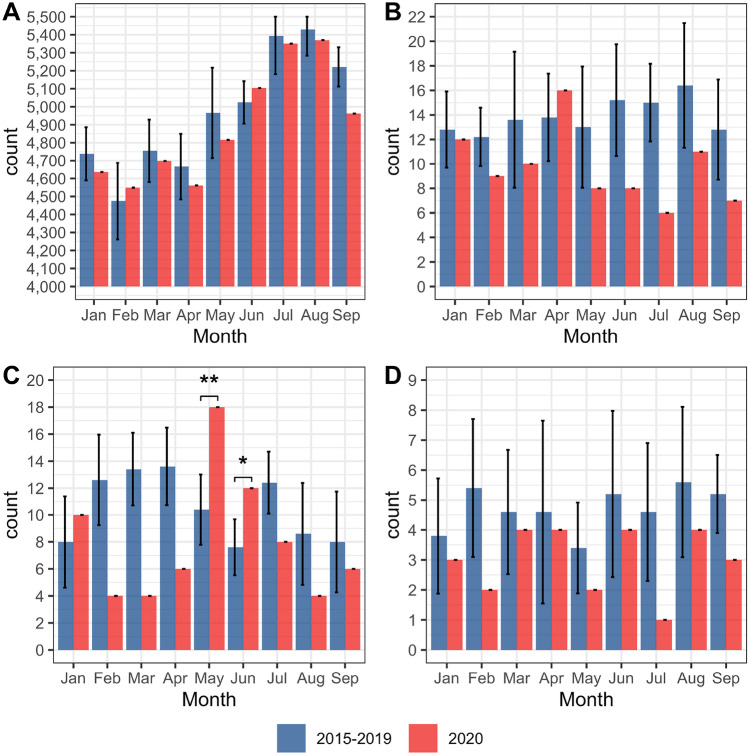
A total of 214,862 births with gestational age ≥ 22 + 0 weeks were registered in Denmark during the period February 27–September 30, 2015–2020. The total stillbirth rate was reduced (OR 0.69, 0.50–0.95), resulting in a corresponding reduction in perinatal mortality (OR 0.76, 0.20–0.98) (Table 1). The very early and early neonatal mortality rates were not different between 2020 and 2015–2019 (Table 1). The live birth rate, for all PTB groups, was not different in 2020 (Table 2). Furthermore, the proportion of term pregnancies was increased (OR 1.38, 1.29–1.46), whereas the proportion of late-term pregnancies was reduced (OR 0.85, 0.71–1.01) (Table 2). The number of live births in 2020 was similar to the mean number of live birth per years during 2015–2019 (Fig. 2A), but the number of stillbirths in 2020 was reduced in all months, except April (Fig. 2B). The number of extremely preterm live births was reduced, albeit insignificantly, in all months, except May (OR 2.72, 1.40–5.09) and June (OR 2.33, 1.05–4.83) where they were significantly increased (Fig. 2C). The number of extremely preterm stillbirths was uniformly reduced in all months (Fig. 2D).
Fig. 2.
Mean number of live births and stillbirths per month, from January to September. 2015–2019 (blue; error bars represent 1SD) and 2020 (red). A Live births (all gestational ages), B stillbirths (all gestational ages), C live births (extremely preterm, gestational age 22–28 weeks), and D stillbirths (gestational age 22–28 weeks). Statistically significant differences are indicated by asterisks. *represents p ≤ 0.05, and ** represents p < 0.01
Combined extremely preterm live and stillbirth rates
The monthly combined stillbirth and live birth rates for infants delivered with gestational age between 22 + 0 and 28 + 0 weeks (extremely preterm) are illustrated in Fig. 3. Despite a non-significant increase in May and June, the combined xPTB rate (live and still) during the COVID-19 restriction period was significantly reduced (2.8 per 1000 births compared to 3.8 per 1000 births in 2015–2019) (OR 0.73, 0.59–0.96). The increase in May and June is underpinned by an increase in xPTBs (live births) (OR 2.72, 1.40–5.09 and OR 2.33, 1.05–4.83), respectively (Fig. 2C). May and June are months where restrictions were eased (Fig. 1A in the supplement) resulting in a marked increase in workplace attendance, public transport use and visits to retail spaces alongside a reduction in time spent in places of residence (Figs. 1C and 2 in the supplement).
Fig. 3.
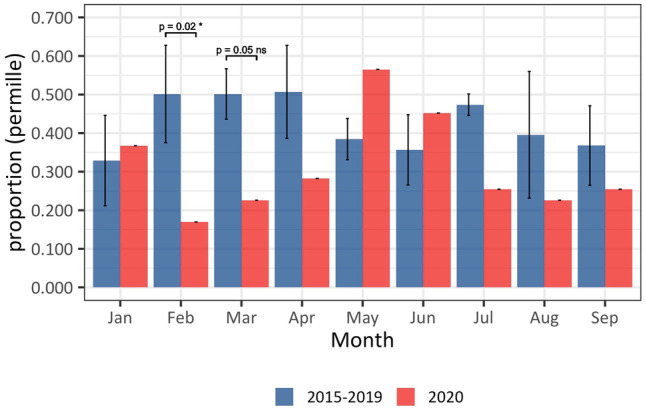
The effect of COVID-19 restrictions on combined extremely preterm stillbirth and live birth rates. Combined rates for 2020 (red columns) are compared to the aggregate rates from the same months in 2015–2019 (blue columns; error bars represent 1SD). The sum of the rates is significantly decreased in February and March 2020. Furthermore, the combined rate is decreased in all months except May and June 2020
Discussion
Prematurity is a complex and challenging pathophysiological condition [27] which is the leading cause of death in children under 5 years old [28, 29]. The complex and multifaceted aetiology of PTB is linked to a wide range of psychosocial, medical and environmental factors, but remains only partly understood [30]. Emerging data suggest that the COVID-19 pandemic and subsequent mitigation measures have provided a natural experiment, through which we can compare PTB and perinatal mortality rates against the human psychosocial and environmental characteristics of pre-COVID-19 and COVID-19 restriction periods.
Using population-based Danish register data, we have documented that the strict lockdown period is associated with a ~ 70% reduction in singleton xPTBs, while no difference was noted for the other PTB groups (Table 2). This result is compatible with the previously reported ~ 90% reduction in xPTBs assessed from the Danish Neonatal Screening Biobank (DNSB) [3], taking into account that three out of four extremely preterm infants died during the first day of life (very early neonatal death)—precluding their appearance in the DNSB (Table 1). The proportion of very PTBs was slightly, ~ 20%, reduced, but there is no evidence for a protective effect of the lockdown in this group. It is important to note that the reduction in xPTBs during the lockdown period does not appear to be mirrored by an increase in stillbirths (Tables 1 and 2). As several studies had reported an increase in stillbirths [5, 13, 15] during the pandemic, a decrease in xPTBs could have been explained by an increase in stillbirth [20]. This does not seem to have been the case in Denmark.
A reduction in PTB or xPTBs has been observed in three nationwide studies from high-income countries (The Netherlands, the USA and Denmark [1, 3, 9]). The stringency of lockdown in all three countries was greater than 70% (Figs. 1, 3, and 5 in the Supplement), with clear effects on location behaviour, e.g., workplace attendance reduced around 50% (Figs. 1-6 in the Supplement). A nationwide study conducted in Sweden found no effect on the rate of PTB [16]; however, the Swedish COVID-19 response was characterized by comparatively lenient lockdown measures, with a stringency of approximately 65% (Fig. 7 in the Supplement). Mobility data from Sweden reflects these measures with an average reduction of workplace attendance of approximately 30% (Figs. 7, 8 in the Supplement). These data suggest that the reduction in xPTBs correlates with the nature and extent of the lockdown, and, in consequence, the behavioural and psychosocial changes associated with an effective lockdown.
The data presented in the current study covers a broader period of the Danish COVID-19 restrictions and demonstrates the temporal variation in xPTB rates after the easing of the lockdown (April 15–September 30) as illustrated by a marked increase in xPTB rates in May and June (Fig. 2C) followed by reduced levels in July, August and September. The net result is that for the whole period with COVID-19 restrictions, the odds ratio of xPTB was reduced, albeit not significantly (Table 2). The months May and June coincide with a reopening of society and a relative return to normal activities (Figs. 1C, 2 in the supplement). For example, the lockdown is characterized by a stable ~ 50% reduction in workplace attendance, during May and June workplace attendance climbs steadily to ~ 20% reduction where, with the exception of the vacation period in July, it remains relatively stable. Thus, the periods characterized by high stringency and considerable mobility reduction (lockdown) correlate with a significant reduction in xPTB, whereas the lockdown easing phase (May and June) coincides with a significant increase in xPTB rate.
The stillbirth rate for the whole COVID-19 restriction period (February 27–September 30) was reduced by ~ 30% (Table 1). The monthly number of total stillbirths (Fig. 2B) and stillbirths among extremely preterm pregnancies (Fig. 2D) do indicate a uniform reduction in the number of stillbirths in each month during the COVID-19 restriction period. Consequently, the stillbirth rate does not account for the reduction in xPTBs during the Danish lockdown and is itself reduced as a possibly unintended beneficial consequence of the COVID-19 restrictions generally.
The reduction in combined extremely preterm stillbirth and live birth rates is statistically significant in February and March, while the increase in May and June is not (Fig. 3). This seems to reflect that the improvement in perinatal health started prior to the lockdown, during which period the Danish government communicated frequently with the public regarding the risk of COVID-19 in Denmark and societal preparedness (Fig. 1) [31].
Perinatal mortality was moderately reduced during the lockdown period and was, as a result of a considerably reduced stillbirth rate, significantly reduced over the extended COVID-19 restriction period (Table 1). Early, and very early, neonatal mortality was not changed during these periods (Table 1). Consequently, despite the strain COVID-19 is expected to place on health care resources [32], the observed reduction of xPTB is unlikely to be driven by a change in obstetric policies, formal or otherwise.
One may speculate on the causes of the reduction in xPTBs and stillbirths—one suggestion is a reduction in exposure to harmful pathogens [33] or changes in genital tract microbiota due to hygiene precautions; massive reductions in the transmission of meningitis [34] and pertussis [35] and an absent influenza season 2020–2021 [36] are consistent with this argument. Furthermore, the number of laboratory-confirmed chlamydia cases dropped ~ 25% during the lockdown compared to the 2015–2019 average, followed by an increase in June and July 2020, and a return to the 2015–2019 level in August [37]. However, the number of people tested for chlamydia during this time was similarly reduced. As a surrogate marker of ascending urogenital infections and microbiota changes, this moderate reduction during the lockdown suggests that a reduction in ascending infections is unlikely to be responsible for the reduction of xPTBs. Exposure to environmental pollutants, particularly air pollution, has been associated with PTB [38]. However, the Danish lockdown was not associated with major changes in the level of air pollution [39].
Reduced anxiety and stress may also play a role by influencing the expression of the chaperone protein FKBP51, which increases in response to stress and alters the oestrogen/progesterone ratio thus overcoming the progesterone effect which inhibits parturition [40, 41]. Two, albeit nearly 30 years old, Danish studies showed an association between anxiety in gestational week 30, but not in week 16, and PTB [42], and an association between very early PTB (< 34 weeks) and severe stressful events in mid-pregnancy [43], respectively. Furthermore, leisure time activity during pregnancy has been associated with a reduced risk of PTB [44], while hard work and hard physical activity during pregnancy could increase the risk of PTB [45, 46]. An Irish questionnaire-based COVID-19 study among 71 pregnant women [47] revealed an increased worry for elderly relatives and children rather than for their pregnancy. Attitudes to the COVID-19 lockdown are being systematically studied as part of the HOPE project [48] in Denmark, and the feeling of being efficacious, i.e., being able to act on information given, seems to be a very significant predictor of protective behaviour [49] in Western democracies. Surprisingly, the lockdown-associated suspension of daily duties has been shown to be of value for people with vulnerable psyches [50]. Thus, in Denmark, the reduced workplace and social stress may outweigh the negative effects of worries associated with the pandemic. While the end of the lockdown period was associated with improved psychological well-being [51], the practicalities of easing lockdown restrictions and returning to work, returning children to school, etc., could increase stress and anxiety. This is particularly true among women with depression (prevalence of 14.2% among Danish women) or anxiety (prevalence of 6.8% among Danish women) [52], both of which have been associated with PTB [53]. We cannot exclude that the psychosocial and behavioural changes during the lockdown, i.e. more people working from home, could have affected the risk of xPTB in specific groups of pregnant women.
The observational nature of this study precludes causal inference, but the sizeable effect on the xPTB rate indicates that it will be worthwhile to identify the elements of the lockdown that have conferred this unintended protective effect regarding xPTB. One might speculate that it will be possible to identify a specific psychosocial phenotype in mid-pregnancy that may benefit from specialized care. However, the complex causality of PTB [30] suggests that defining controllable protective factors will require a cross-disciplinary effort.
The use of national registers allowed us to examine all Danish pregnancies, thus avoiding selection bias and allowing us to provide a complete assessment of birth and mortality rates.
Conclusion
The period of COVID-19 restrictions was characterized by a reduction in the extremely preterm live birth rate, in the strict lockdown period, and a reduction in the stillbirth rate throughout the extended period of restrictions. Furthermore, there was a tendency for pregnancies to run to term during this period.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
This research was conducted using data obtained from the Danish National registers. The Danish Health Data Authority is acknowledged for its support with preparation and linkage of the data.
Abbreviations
- CI
Confidence interval
- DNSB
Danish Neonatal Screening Biobank
- LMIC
Low- to middle-income countries
- LPR
Danish National Patient Register
- LPR2
An older version of LPR
- LPR3
Most recent version of LPR, implemented from February 2019
- N
Population size
- OR
Odds ratio
- PTB
Preterm birth
- SD
Standard deviation
- xPTB
Extremely PTB
Author contribution
P.L.H. and M.C.: conceptualized and designed the study, analysed and interpreted the data, drafted and edited the initial manuscript and reviewed and revised the manuscript. G.H. and U.L.T.: participated in the conceptualized and designed the study, co-wrote and revised the article, checked all the processes of the article. C.M.H., M.B.H., S.H., J.S.J.: curated and interpreted the data and revised the article. H.H., K.R., A.D.L., A.H.: developed the methodology and revised the article. M.B., D.M.H., L.K.: administered the project and revised the article. M.M.: revised the article. All authors approved the final manuscript for submission and agree to be accountable for all aspects of the work. P.L.H. and G.H. equally contributed to this work as first authors and M.C. and U.L.T. equally contributed as corresponding authors.
Availability of data and material
Register data is available for research purposes, following authorization, through the usual channels.
Code availability
Not applicable.
Declarations
Ethics approval
The study was conducted according to Danish legislation and guidelines for register research and was approved by the Data Protection Agency officer at Statens Serum Institut (No: 20/04753).
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
Dr Breindahl has a patent (NeoHelp) with royalties paid. Dr Breindahl has nothing else to disclose. All other authors reported to have nothing to disclose.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Paula L. Hedley and Gitte Hedermann contributed equally as first authors. Ulrik Lausten-Thomsen and Michael Christiansen contributed equally as corresponding authors.
Contributor Information
Ulrik Lausten-Thomsen, Email: ulrik.lausten-thomsen@regionh.dk.
Michael Christiansen, Email: mic@ssi.dk.
References
- 1.Been JV, Burgos Ochoa L, Bertens LCM, Schoenmakers S, Steegers EAP, Reiss IKM. Impact of COVID-19 mitigation measures on the incidence of preterm birth: a national quasi-experimental study. Lancet Public Health. 2020;5:e604–e611. doi: 10.1016/S2468-2667(20)30223-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goldenberg RL, McClure EM. Have coronavirus disease 2019 (COVID-19) community lockdowns reduced preterm birth rates? Obstet Gynecol. 2021;137:399–402. doi: 10.1097/AOG.0000000000004302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hedermann G, Hedley PL, Baekvad-Hansen M, Hjalgrim H, Rostgaard K, Poorisrisak P, Breindahl M, Melbye M, Hougaard DM, Christiansen M, Lausten-Thomsen U. Danish premature birth rates during the COVID-19 lockdown. Arch Dis Child Fetal Neonatal Ed. 2021;106:93–95. doi: 10.1136/archdischild-2020-319990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maeda Y, Nakamura M, Ninomiya H, Ogawa K, Sago H, Miyawaki A. Trends in intensive neonatal care during the COVID-19 outbreak in Japan. Arch Dis Child Fetal Neonatal Ed. 2021;106:327–329. doi: 10.1136/archdischild-2020-320521. [DOI] [PubMed] [Google Scholar]
- 5.De Curtis M, Villani L, Polo A (2020) Increase of stillbirth and decrease of late preterm infants during the COVID-19 pandemic lockdown. Arch Dis Child Fetal Neonatal Ed 106:456 [DOI] [PMC free article] [PubMed]
- 6.Harvey EM, McNeer E, McDonald MF, Shapiro-Mendoza CK, Dupont WD, Barfield W, Patrick SW. Association of preterm birth rate with COVID-19 statewide stay-at-home orders in Tennessee. JAMA Pediatr. 2021;175:635–637. doi: 10.1001/jamapediatrics.2020.6512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Richter F, Strasser AS, Suarez-Farinas M, Zhao S, Nadkarni GN, Jabs EW, Guttmann K, Glicksberg BS (2021) Neonatal outcomes during the COVID-19 pandemic in New York City. Pediatr Res. 10.1038/s41390-021-01513-7 [DOI] [PMC free article] [PubMed]
- 8.Meyer R, Bart Y, Tsur A, Yinon Y, Friedrich L, Maixner N, Levin G. A marked decrease in preterm deliveries during the coronavirus disease 2019 pandemic. Am J Obstet Gynecol. 2021;224:234–237. doi: 10.1016/j.ajog.2020.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gemmill A, Casey JA, Catalano R, Karasek D, Bruckner T (2021) Changes in live births, preterm birth, low birth weight, and cesarean deliveries in the United States during the SARS-CoV-2 pandemic. medRXiv 2021.03.20.21253990 [DOI] [PMC free article] [PubMed]
- 10.Philip RK, Purtill H, Reidy E, Daly M, Imcha M, McGrath D, O’Connell NH, Dunne CP (2020) Unprecedented reduction in births of very low birthweight (VLBW) and extremely low birthweight (ELBW) infants during the COVID-19 lockdown in Ireland: a ‘natural experiment’ allowing analysis of data from the prior two decades. BMJ Glob Health 5:e003075 [DOI] [PMC free article] [PubMed]
- 11.Main EK, Chang SC, Carpenter AM, Wise PH, Stevenson DK, Shaw GM, Gould JB. Singleton preterm birth rates for racial and ethnic groups during the coronavirus disease 2019 pandemic in California. Am J Obstet Gynecol. 2021;224:239–241. doi: 10.1016/j.ajog.2020.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Handley SC, Mullin AM, Elovitz MA, Gerson KD, Montoya-Williams D, Lorch SA, Burris HH. Changes in preterm birth phenotypes and stillbirth at 2 Philadelphia hospitals during the SARS-CoV-2 pandemic, March-June 2020. JAMA. 2021;325:87–89. doi: 10.1001/jama.2020.20991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mor M, Kugler N, Jauniaux E, Betser M, Wiener Y, Cuckle H, Maymon R. Impact of the COVID-19 pandemic on excess perinatal mortality and morbidity in Israel. Am J Perinatol. 2021;38:398–403. doi: 10.1055/s-0040-1721515. [DOI] [PubMed] [Google Scholar]
- 14.Arnaez J, Ochoa-Sangrador C, Caserio S, Gutierrez EP, Jimenez MDP, Castanon L, Benito M, Pena A, Hernandez N, Hortelano M, Schuffelmann S, Prada MT, Diego P, Villagomez FJ, Garcia-Alix A. Lack of changes in preterm delivery and stillbirths during COVID-19 lockdown in a European region. Eur J Pediatr. 2021;180:1997–2002. doi: 10.1007/s00431-021-03984-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khalil A, von Dadelszen P, Draycott T, Ugwumadu A, O’Brien P, Magee L (2020) Change in the incidence of stillbirth and preterm delivery during the COVID-19 pandemic. JAMA 324:705–706 [DOI] [PMC free article] [PubMed]
- 16.Pasternak B, Neovius M, Soderling J, Ahlberg M, Norman M, Ludvigsson JF, Stephansson O (2021) Preterm birth and stillbirth during the COVID-19 pandemic in Sweden: a nationwide cohort study. Ann Intern Med 174:873–875 [DOI] [PMC free article] [PubMed]
- 17.Chmielewska B, Barratt I, Townsend R, Kalafat E, van der Meulen J, Gurol-Urganci I, O’Brien P, Morris E, Draycott T, Thangaratinam S, Le Doare K, Ladhani S, von Dadelszen P, Magee L, Khalil A. Effects of the COVID-19 pandemic on maternal and perinatal outcomes: a systematic review and meta-analysis. Lancet Glob Health. 2021;9:e759–e772. doi: 10.1016/S2214-109X(21)00079-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kc A, Gurung R, Kinney MV, Sunny AK, Moinuddin M, Basnet O, Paudel P, Bhattarai P, Subedi K, Shrestha MP, Lawn JE, Malqvist M. Effect of the COVID-19 pandemic response on intrapartum care, stillbirth, and neonatal mortality outcomes in Nepal: a prospective observational study. Lancet Glob Health. 2020;8:e1273–e1281. doi: 10.1016/S2214-109X(20)30345-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rasmussen MI (2020) Extremely preterm infant admissions within the SafeBoosC-III consortium during the COVID-19 lockdown. Front Pediatr 9:647880 [DOI] [PMC free article] [PubMed]
- 20.Homer CSE, Leisher SH, Aggarwal N, Akuze J, Babona D, Blencowe H, Bolgna J, Chawana R, Christou A, Davies-Tuck M, Dandona R, Gordijn S, Gordon A, Jan R, Korteweg F, Maswime S, Murphy MM, Quigley P, Storey C, Vallely LM, Waiswa P, Whitehead C, Zeitlin J, Flenady V. Counting stillbirths and COVID 19-there has never been a more urgent time. Lancet Glob Health. 2021;9:e10–e11. doi: 10.1016/S2214-109X(20)30456-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schmidt M, Schmidt SAJ, Adelborg K, Sundboll J, Laugesen K, Ehrenstein V, Sorensen HT. The Danish health care system and epidemiological research: from health care contacts to database records. Clin Epidemiol. 2019;11:563–591. doi: 10.2147/CLEP.S179083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schmidt M, Pedersen L, Sorensen HT. The Danish Civil Registration System as a tool in epidemiology. Eur J Epidemiol. 2014;29:541–549. doi: 10.1007/s10654-014-9930-3. [DOI] [PubMed] [Google Scholar]
- 23.The Danish Medical Birth Registry. https://www.danishhealthdata.com/find-health-data/Det-medicinske-foedselsregister [PubMed]
- 24.Schmidt M, Schmidt SA, Sandegaard JL, Ehrenstein V, Pedersen L, Sorensen HT. The Danish National Patient Registry: a review of content, data quality, and research potential. Clin Epidemiol. 2015;7:449–490. doi: 10.2147/CLEP.S91125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hale T, Angrist N, Goldszmidt R, Kira B, Petherick A, Phillips T, Webster S, Cameron-Blake E, Hallas L, Majumdar S, Tatlow H. A global panel database of pandemic policies (Oxford COVID-19 Government Response Tracker) Nat Hum Behav. 2021;5:529–538. doi: 10.1038/s41562-021-01079-8. [DOI] [PubMed] [Google Scholar]
- 26.Google (2021) Google COVID-19 Community Mobility Reports. https://www.google.com/covid19/mobility/
- 27.Saigal S, Doyle LW. An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet. 2008;371:261–269. doi: 10.1016/S0140-6736(08)60136-1. [DOI] [PubMed] [Google Scholar]
- 28.Chawanpaiboon S, Vogel JP, Moller AB, Lumbiganon P, Petzold M, Hogan D, Landoulsi S, Jampathong N, Kongwattanakul K, Laopaiboon M, Lewis C, Rattanakanokchai S, Teng DN, Thinkhamrop J, Watananirun K, Zhang J, Zhou W, Gulmezoglu AM. Global, regional, and national estimates of levels of preterm birth in 2014: a systematic review and modelling analysis. Lancet Glob Health. 2019;7:e37–e46. doi: 10.1016/S2214-109X(18)30451-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harrison MS, Goldenberg RL. Global burden of prematurity. Semin Fetal Neonatal Med. 2016;21:74–79. doi: 10.1016/j.siny.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 30.Romero R, Dey SK, Fisher SJ. Preterm labor: one syndrome, many causes. Science. 2014;345:760–765. doi: 10.1126/science.1251816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Christensen JG, Askim J, Gyrd-Hansen D, Østergaard L (2021) [The handling of covid-19 in the spring of 2020 - Report submitted by the investigation group set up by the Danish Government’s Committee on the Rules of Procedure regarding the handling of covid-19.]. Folketinget, Copenhagen, Denmark
- 32.Sinard JH. An analysis of the effect of the COVID-19 pandemic on case volumes in an academic subspecialty-based anatomic pathology practice. Acad Pathol. 2020;7:2374289520959788. doi: 10.1177/2374289520959788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cunnington M, Kortsalioudaki C, Heath P. Genitourinary pathogens and preterm birth. Curr Opin Infect Dis. 2013;26:219–230. doi: 10.1097/QCO.0b013e328360dc31. [DOI] [PubMed] [Google Scholar]
- 34.SSI (2021) No 3/5 - 2021: Pneumococcal vaccine for vaccination of risk group patients now again open for orders; considerable drop in invasive meningococcal disease in connection with the 2020 lock down. EPI-NEWS. Statens Serum Institut, Copenhagen, Denmark
- 35.SSI (2020) No 27/33 - 2020: Laboratory-confirmed whooping cough 2019. EPI-NEWS. Statens Serum Institut, Copenhagen, Denmark
- 36.SSI (2021) No 2 - 2021: This year’s flu is still waiting under coronavirus restrictions. INFLUENZA-NEWS. Statens Serum Institut, Copenhagen, Denmark
- 37.Hedley PL, Hoffmann S, Lausten-Thomsen U, Voldstedlund M, Bjerre KD, Hviid A, Krebs L, Jensen JS, Christiansen M (2021) A nationwide examination of Chlamydia trachomatis infections in Denmark throughout the COVID-19 pandemic. medRXiv 2021.06.30.21259819 [DOI] [PMC free article] [PubMed]
- 38.Bekkar B, Pacheco S, Basu R, DeNicola N (2020) Association of Air Pollution and Heat Exposure With Preterm Birth, Low Birth Weight, and Stillbirth in the US: A Systematic Review. JAMA Netw Open 3:e208243 [DOI] [PMC free article] [PubMed]
- 39.European Environment Agency (2020) Air quality and COVID-19. Air pollution. European Environment Agency, Copenhagen, Denmark
- 40.Guzeloglu-Kayisli O, Semerci N, Guo X, Larsen K, Ozmen A, Arlier S, Mutluay D, Nwabuobi C, Sipe B, Buhimschi I, Buhimschi C, Schatz F, Kayisli UA, Lockwood CJ (2021) Decidual cell FKBP51-progesterone receptor binding mediates maternal stress-induced preterm birth. Proc Natl Acad Sci USA 118:e2010282118 [DOI] [PMC free article] [PubMed]
- 41.Li H, Su P, Lai TK, Jiang A, Liu J, Zhai D, Campbell CT, Lee FH, Yong W, Pasricha S, Li S, Wong AH, Ressler KJ, Liu F. The glucocorticoid receptor-FKBP51 complex contributes to fear conditioning and posttraumatic stress disorder. J Clin Invest. 2020;130:877–889. doi: 10.1172/JCI130363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hedegaard M, Henriksen TB, Sabroe S, Secher NJ. Psychological distress in pregnancy and preterm delivery. BMJ. 1993;307:234–239. doi: 10.1136/bmj.307.6898.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lou HC, Nordentoft M, Jensen F, Pryds O, Nim J, Hemmingsen R. Psychosocial stress and severe prematurity. Lancet. 1992;340:54. doi: 10.1016/0140-6736(92)92468-U. [DOI] [PubMed] [Google Scholar]
- 44.Aune D, Schlesinger S, Henriksen T, Saugstad OD, Tonstad S. Physical activity and the risk of preterm birth: a systematic review and meta-analysis of epidemiological studies. BJOG. 2017;124:1816–1826. doi: 10.1111/1471-0528.14672. [DOI] [PubMed] [Google Scholar]
- 45.Saurel-Cubizolles MJ, Kaminski M, Llado-Arkhipoff J, Du Mazaubrun C, Estryn-Behar M, Berthier C, Mouchet M, Kelfa C. Pregnancy and its outcome among hospital personnel according to occupation and working conditions. J Epidemiol Community Health. 1985;39:129–134. doi: 10.1136/jech.39.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Teitelman AM, Welch LS, Hellenbrand KG, Bracken MB. Effect of maternal work activity on preterm birth and low birth weight. Am J Epidemiol. 1990;131:104–113. doi: 10.1093/oxfordjournals.aje.a115463. [DOI] [PubMed] [Google Scholar]
- 47.Corbett GA, Milne SJ, Hehir MP, Lindow SW, O’Connell MP. Health anxiety and behavioural changes of pregnant women during the COVID-19 pandemic. Eur J Obstet Gynecol Reprod Biol. 2020;249:96–97. doi: 10.1016/j.ejogrb.2020.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.HOPE (2021) How democracies Cope with Covid19. Århus University
- 49.Jorgensen F, Bor A, Petersen MB. Compliance without fear: individual-level protective behaviour during the first wave of the COVID-19 pandemic. Br J Health Psychol. 2021;26:679–696. doi: 10.1111/bjhp.12519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rasmussen GV, Garbers C, D. Psykisk sårbarhed og kalenderens opløsning under Corona. Jordens Folk. 2020;56:55–67. [Google Scholar]
- 51.Sonderskov KM, Dinesen PT, Santini ZI, Ostergaard SD. Increased psychological well-being after the apex of the COVID-19 pandemic. Acta Neuropsychiatr. 2020;32:277–279. doi: 10.1017/neu.2020.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Munk-Jorgensen P, Allgulander C, Dahl AA, Foldager L, Holm M, Rasmussen I, Virta A, Huuhtanen MT, Wittchen HU. Prevalence of generalized anxiety disorder in general practice in Denmark, Finland, Norway, and Sweden. Psychiatr Serv. 2006;57:1738–1744. doi: 10.1176/ps.2006.57.12.1738. [DOI] [PubMed] [Google Scholar]
- 53.Staneva A, Bogossian F, Pritchard M, Wittkowski A. The effects of maternal depression, anxiety, and perceived stress during pregnancy on preterm birth: a systematic review. Women Birth. 2015;28:179–193. doi: 10.1016/j.wombi.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 54.Danish Health Authority (2020) Guidelines for handling covid-19. Danish Health Authority, March 4
- 55.Danish Health Authority (2020) COVID-19 Risk assessment, strategy and measures in the event of an epidemic in Denmark. Sundhedsstyrelsen, Copenhagen, Denmark
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Register data is available for research purposes, following authorization, through the usual channels.
Not applicable.