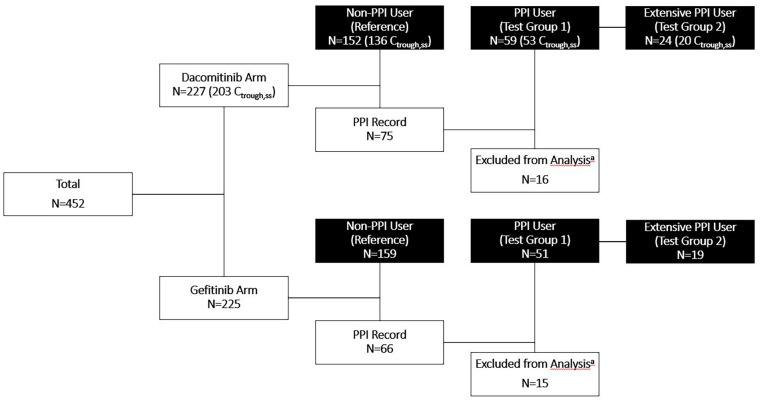
Fig. 1.
Summary of patients grouped by PPI use in the intent-to-treat population randomized to dacomitinib or gefitinib treatment arms. Black boxes represent groups used in analyses. For the dacomitinib arm, the numbers of patients with evaluable Ctrough for PK analysis are included in the parentheses. PPI Record indicates any patient who had a PPI record. aSixteen patients in the dacomitinib arm and 15 patients in the gefitinib arm were not included in the analysis. These patients either only had a PPI record after stopping dacomitinib or gefitinib treatment, or their PPI records had no start or end date, making it impossible to determine whether those patients took PPIs concurrently with study treatment. PPI proton pump inhibitor; Ctrough,ss steady-state trough concentration