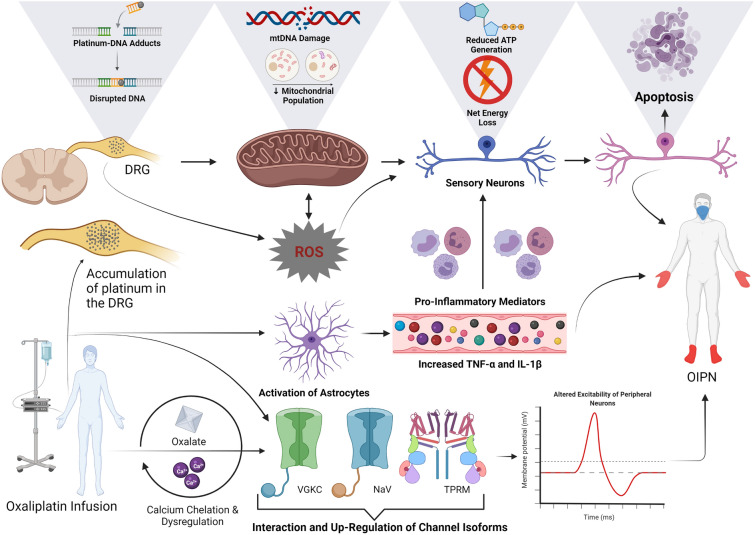
Fig. 1.
The current hypothesis for the pathogenesis of OIPN. Accumulation of oxaliplatin occurs in dorsal root ganglion neurons, where it interferes with DNA and mtDNA cross-linking. This results in a direct dose-dependent toxicity of DRG neurons and neuronal mitochondria. There is a subsequent decrease in mitochondrial respiration and ATP. The resultant oxidative stress contributes to disruption in DNA and mtDNA replication and transcription, leading to diminished energy status and increased neuronal apoptosis. Increased production of ROS together with activation of astrocytes causes the release of pro-inflammatory mediators TNF-α and IL-1β and decreased expression of cytokines IL-10 and IL-4 with a neuroprotective function. Subsequently, leucocytes are activated and travel down a chemotactic gradient to the dorsal root ganglion and peripheral nerves, leading to neuroinflammation. Neuroinflammation and ROS cause damage to dorsal root ganglion neurons, leading to apoptosis, which contributes to calcium dysregulation, axonal energy depletion and damage to neuronal organelles. Both ROS and neuroinflammation are implicated in nociceptor sensitisation, mechanical hyperalgesia and cold allodynia in preclinical models. Oxaliplatin interacts directly with VGKC, NaV channel, TRPM isoforms in sensory neurons contributing to cold hyperalgesia/allodynia and hyperexcitability of peripheral neurons. Further, a metabolite of oxaliplatin, oxalate chelates Ca2+ ions in the acute phase, contributing to neuronal excitability and increasing spontaneous activity of neurons. ATP: adenosine triphosphate, Ca2+: calcium, DNA: deoxyribonucleic acid, DRG: dorsal root ganglion, NaV: voltage-gated sodium, OIPN: oxaliplatin-induced peripheral neuropathy, mtDNA: mitochondrial DNA, ROS: reactive oxygen species, IL-1B: interleukin 1B, IL-4: interleukin 4, IL-10: interleukin 10, TRPM: transient receptor potential melastatin, TNF-α: tumour necrosis factor-α, VGKC: voltage-gated potassium channel