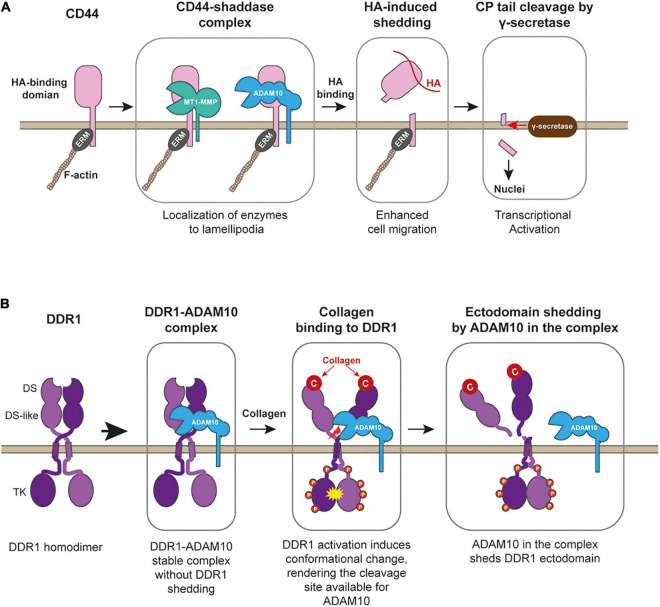
FIGURE 3.
CD44 processing and collagen-induced shedding of DDR1 by ADAM10. (A) CD44 forms stable complex with MT1-MMP (or potentially with ADAM10), allowing the enzymes to localize at lamellipodia. Upon binding to HA-substratum at the leading edge, the enzyme in the complex shed the ectodomain of CD44, releasing CD44-mediated cell adhesion and promote cell migration. After ectodomain removal, γ-secretase releases the cytoplamic tail of the CD44, and released cytoplasmic tail acts as a transcriptional factor to stimulate gene expression. ERM, band 3.1 proteins. (B) DDR1 upon secretion forms a stable complex with ADAM10, but ADAM10 does not cleave DDR1. Collagen binding activates DDR1 and induces conformational change in its ectodomain, rendering the cleavage site available to ADAM10. ADAM10 cleaves between Pro407-Val408 at the juxtamembrane region to release DDR1-dependent cell adhesion and to stop collagen signaling through DDR1.