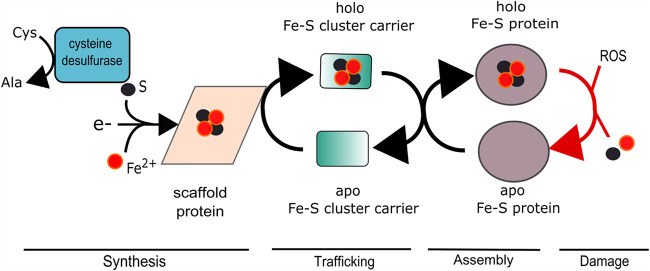
FIG 1.
General mechanism of bacterial Fe-S protein assembly. Monoatomic Fe2+ and S0 are combined with electrons on a proteinaceous molecular scaffold forming an Fe-S cluster. The Fe-S cluster is transferred to one or more carrier proteins before being transferred to an apo-protein forming a holo-protein. Reactive oxygen species (ROS) can either damage the Fe-S cluster, which can subsequently be repaired, or destroy it, resulting in apo-protein formation.