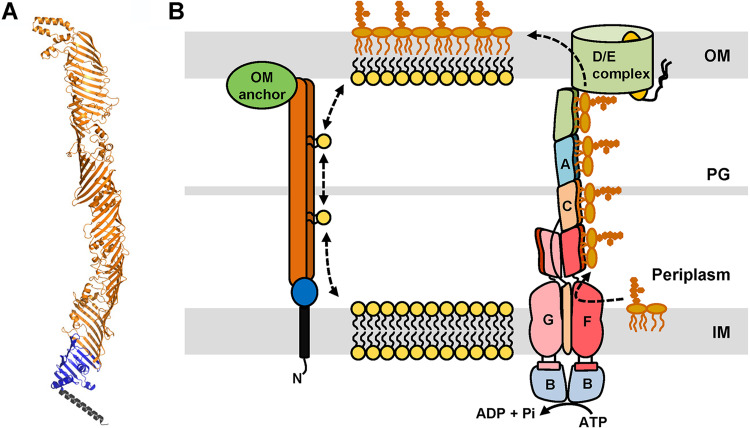
FIG 6.
Model of OM lipid assembly. (A) Cartoon representation of the structural prediction for YhdP by AlphaFold (32). Regions of the structural model are colored as in Fig. 1A, with the transmembrane helix in black, the chorein-N domain in blue, and the AsmA domain in orange. (B) Model of OM lipid transport and assembly. The Lpt system (on the right) transports newly synthesized LPS molecules from the IM to the outer leaflet of the OM. We propose that TamB, YhdP, and YdbH transport phospholipids between the IM and OM. In this model, TamB, YhdP, and YdbH physically bridge the IM and OM by anchoring to the IM through their N-terminal α-helix (black segment) and likely to the OM via interactions with partners (green oval). The predicted structure of the large periplasmic region of these proteins would form a structure similar to the Lpt bridge and provide protection to the hydrophobic fatty acid chains of phospholipids as they travel through the periplasm. LPS transport is unidirectional and powered by ATP, while bidirectional phospholipid transport would be driven by diffusion through TamB, YhdP, and YdbH.