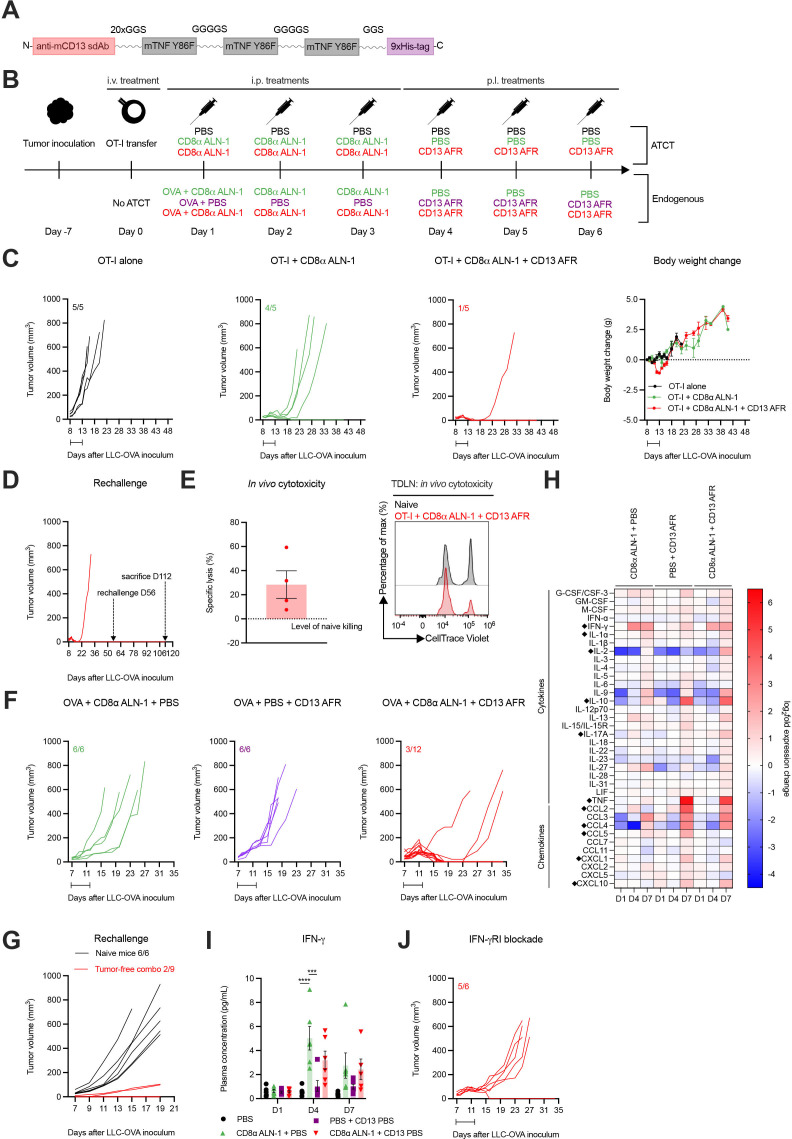
Figure 7.
Strong synergy between CD8α ALN-1 and neovasculature-targeted TNF mediates complete tumor eradication in the absence of systemic toxicity and allows for immunological protection against secondary tumor challenge. (A) Molecular TNF AcTakine design. (B) Mice with LLC-OVA tumors received OT-I CD8+ T cell transfer (top) or not (bottom) and were treated with PBS or CD8α ALN-1 (10 µg) in combination with PBS or CD13 AFR (50 µg) (top). Mice not treated with ATCT (bottom) received OVA (100 µg) combined with treatments described above (top). (C) LLC-OVA tumor growth (left) and change in body weight (right) (ATCT). Lines represent individual mice. Data points represent the mean ± SEM of an experiment with n=5 mice/group. (D) LLC-OVA tumor growth (ATCT, rechallenge). (E) Cytotoxicity towards SIINFEKL+ cells. Bar represents the mean ± SEM of an experiment with n=4 mice/group (left) with representative flow cytometry histograms (right). (F) LLC-OVA tumor growth (without ATCT). Lines represent individual mice of an experiment with n=6 or 12 mice/group. (G) LLC-OVA tumor growth (without ATCT, rechallenge). (H) Heat map of log2-fold changes in plasma cytokine and chemokine levels. Group means of an experiment with n=6 mice/group. Statistically significant differences are indicated with a diamont icon. (I) Plasma IFN-γ concentration. Bars represent the mean ± SEM of an experiment with n=6 mice/group. ***p<0.001, ****p<0.0001; ns, p≥0.05 by one-way ANOVA with Tukey’s multiple comparisons test. (J) LLC-OVA tumor growth. Mice were treated with an anti-mouse IFN-γRI antibody (500 µg) every 48 hours starting 1 day before the first CD8α ALN-1 delivery. Intervals indicate the treatment periods. See also online supplemental figures 14–16. AcTakine, Activity-on-Target cytokine; ANOVA, analysis of variance; ATCT, adoptive T cell transfer; IFN-γ, interferon-γ; IL, interleukin; LLC, Lewis lung carcinoma; ns, not significant; OVA, ovalbumin; TNF, tumor necrosis factor.