Abstract
Objectives
To estimate the frequency of chronic conditions and geriatric syndromes in older patients admitted to hospital because of an exacerbation of their chronic conditions, and to identify multimorbidity clusters in these patients.
Design
Multicentre, prospective cohort study.
Setting
Internal medicine or geriatric services of five general teaching hospitals in Spain.
Participants
740 patients aged 65 and older, hospitalised because of an exacerbation of their chronic conditions between September 2016 and December 2018.
Primary and secondary outcome measures
Active chronic conditions and geriatric syndromes (including risk factors) of the patient, a score about clinical management of chronic conditions during admission, and destination at discharge were collected, among other variables. Multimorbidity patterns were identified using fuzzy c-means cluster analysis, taking into account the clinical management score. Prevalence, observed/expected ratio and exclusivity of each chronic condition and geriatric syndrome were calculated for each cluster, and the final solution was approved after clinical revision and discussion among the research team.
Results
740 patients were included (mean age 84.12 years, SD 7.01; 53.24% female). Almost all patients had two or more chronic conditions (98.65%; 95% CI 98.23% to 99.07%), the most frequent were hypertension (81.49%, 95% CI 78.53% to 84.12%) and heart failure (59.86%, 95% CI 56.29% to 63.34%). The most prevalent geriatric syndrome was polypharmacy (79.86%, 95% CI 76.82% to 82.60%). Four statistically and clinically significant multimorbidity clusters were identified: osteoarticular, psychogeriatric, cardiorespiratory and minor chronic disease. Patient-level variables such as sex, Barthel Index, number of chronic conditions or geriatric syndromes, chronic disease exacerbation 3 months prior to admission or destination at discharge differed between clusters.
Conclusions
In older patients admitted to hospital because of the exacerbation of chronic health problems, it is possible to define multimorbidity clusters using soft clustering techniques. These clusters are clinically relevant and could be the basis to reorganise healthcare circuits or processes to tackle the increasing number of older, multimorbid patients.
Trial registration number
Keywords: internal medicine, statistics & research methods, geriatric medicine, quality in health care
Strengths and limitations of this study.
The multimorbidity analysis in this study has been developed considering a wide range of long-term conditions that may require healthcare in older people.
To the best of our knowledge, this is the first published study of multimorbidity clusters in older patients to include chronic diseases weighted by a clinical management score and geriatric syndromes.
Soft clustering is an innovative, methodologically robust technique that can lead to reliable results in the field of multimorbidity analysis.
The list of chronic conditions and geriatric syndromes used in this study is comprehensive but not standardised, thus hindering comparability with other studies.
Background
According to the most recent Eurostat baseline population projections, old-age dependency ratio (population 65 years and over divided by population 15–64 years) is about 32% in the European Union (EU) and it is expected to reach 52% in 2050, meaning that the EU’s population will continue to grow older.1 Together with the fact that chronic conditions (CC) are the main cause of disability and mortality in Europe, this implies that the coexistence of two or more chronic health conditions, which constitutes the classic definition of multimorbidity, is becoming increasingly common.2
Multimorbidity is therefore turning into an important challenge for the health system because of the expanding proportion of older people with multiple CC and treatments as well as the difficulties associated with their clinical management (CM).3 4 Most clinical practice guidelines are focused on single diseases, with limited recommendations for multimorbid patients,5 and, in addition, randomised clinical trials often exclude older patients with multimorbidity.6 Despite the importance of multimorbidity in clinical practice, different criteria about which conditions should be considered and how to aggregate them are still under debate, which makes it difficult to compare different estimations around the world.7–9
One of the novel, increasingly widespread definitions of multimorbidity considers the non-spurious association of certain CC by sharing pathophysiological mechanisms, giving rise to disease association patterns.10 In order to identify those patterns, different statistical methodologies have been explored. Among these techniques, soft clustering allows to focus on patients rather than diagnoses and is a useful method when there is a high overlap of diagnoses between patients, as it enables patients to belong to more than one multimorbidity pattern with a certain probability.11
Besides CC, other clinically relevant situations such as geriatric syndromes (GS) may also be considered in the definition of these patterns, since they might have a great impact on the health-related quality of life and CM of old patients.12 In fact, the purpose of multimorbidity characterisation (ie, predicting outcomes or use of health services, improving quality of care, organising healthcare services, etc), will have an influence on its definition. In order to have an accurate picture of the morbidity of each patient, a global consideration of all conditions that may require healthcare attention is necessary, even if they are not the reason for hospitalisation. Along these lines, some countries have explicitly recommended to acknowledge all long-term conditions for optimising care of adult patients by reducing, for example, possible inappropriate treatments, multiple healthcare appointments or poor health-related quality of life.13–15
During the past decade, there has been an increasing amount of publications that consider multimorbidity,16 but few have focused on multimorbidity patterns in older patients and even fewer take into account GS.17 For this reason, we launched a multicentre study in 2016 with multiple aims related to multimorbidity, appropriateness of chronic treatments and adverse drug reactions in older patients.18 The objectives of the present analyses were to estimate the frequency of CC and GS in older patients admitted to hospital because of an exacerbation of their CC, and to identify possible multimorbidity patterns in these patients.
Methods
Design and setting
A multicentre, prospective cohort study including older patients hospitalised at the internal medicine or geriatric services at five general teaching hospitals in three different regions of Spain between September 2016 and December 2018 was designed. The detailed protocol was previously published.18
For the purposes of the study, older patients (≥65 years old) admitted as a result of the exacerbation of their chronic pathology were included. Patients referred to home hospitalisation, admitted because of an acute process not related to any chronic disease or with a fatal outcome expected at the time of admission were not included.
No written informed consent was deemed necessary for this study.
Data acquisition and variables
The following sociodemographic and clinical data was retrieved by the clinical team responsible for the patient: patient’s code, date of birth, sex, functional status just before entering the hospital (Barthel Index),19 household (alone, with relatives or other people, in a nursing home), existence of any contact with healthcare services (primary care, emergencies, hospital admission, outpatient care, home care) in the 3 months prior to hospitalisation due to exacerbation of any chronic disease, and destination at discharge from the present episode of hospitalisation (home, transfer to another hospital, transfer to a nursing home, exitus).
Active CC of the patient at arrival to hospital, including some risk factors, were collected (see online supplemental table 1). For this purpose, the physicians of the project defined, on a consensual basis, a limited list of 64 CC, coming from the 114 groups defined by Salisbury et al 20 and including the 19 categories of the Charlson Index.21 Following the same criteria as Salisbury et al, a condition was considered to be chronic when it lasted for at least 6 months, including past conditions that require ongoing disease or risk management, important conditions with a significant risk of recurrence, or past conditions that have continuing implications for patient management.20 Drug-related conditions of this list refer to poor management of medication related to a chronic disease that has clinical implications in that hospitalisation (such as any drug intolerance or an excess drug poisoning).
bmjopen-2021-049334supp001.pdf (120KB, pdf)
Additionally, for each of the CC, it was also recorded if they had required CM (both at admission and during hospitalisation) by assigning a (subjective) correlative score (CM=1, 2, 3…) to each one, according to their clinical importance during the attention process. Thus, CC that did not have any significance during hospitalisation, although recorded, had a score equal to zero. This correlative score was later used to compute a ratio to reflect the weight of each CC in each patient in the index hospitalisation.
Specific GS and risk factors (acute confusional syndrome/delirium, chronic pain, cognitive/intellectual impairment, constipation, depression or anxiety, dysphagia, frailty, immobility, incontinence (urinary/faecal), instability/falls, malnutrition, polypharmacy, pressure ulcers, sensorial deficit, sleep disorders/insomnia) were also recorded. Two of the departments systematically apply a recently developed scale for frailty,22 while the others consider clinical judgement (although based on the same variables).
In order to address potential sources of bias, a pilot study was conducted with the first 10 admissions per centre to validate the data collection process and identify problems that could arise. After that, proper changes were made in the protocol and questionnaire. All available information sources were consulted in order to register CC and GS, and the defined list was not closed. Nonetheless, the registration of CC and GS was based on clinical criteria.
Sampling and analysis
A consecutive sample of 740 patients meeting the inclusion criteria were included, proportionally distributed to the annual volume of hospitalisations of the medicine and/or geriatric services of each centre. The estimated sample of 800 patients (see protocol18) could not be reached due to organisational reasons in one of the participating centres.
For the purposes of the analyses, some CC were grouped according to clinical criteria: Hemiplegia was included in cerebrovascular disease; metastatic solid tumour, leukaemia, lymphoma and any malignancy were grouped into ‘neoplasia’; hepatitis B and C were included in mild liver disease, and both congestive and non-congestive heart failure were grouped into ‘heart failure’. Other diseases were finally excluded of the analyses considering that they have no impact on acute healthcare (cataract, dermatitis, diverticular disease of the colon, glaucoma, haemorrhoids, other vascular diseases and prostatic benign hypertrophy). In the end, 51 CC and 15 GS were analysed.
The updated Charlson Comorbidity Index,23 age adjusted, was computed and categorised according to tertiles distribution.
Descriptive statistics were performed to assess patient clinical and sociodemographic characteristics and to obtain overall prevalence estimates of CC and GS, stratified by sex. Multimorbidity was first defined as the presence of two or more CC. Cumulative number of CC and GS per patient were computed, respectively.
Multimorbidity patterns
CC or GS with a prevalence <2% were excluded to avoid statistical noise and therefore spurious findings in the cluster solutions, leaving a list of 40 CC and 15 GS. In order to take into account if a CC had required CM, a ratio variable (R) was computed as follows:
If CC=0 & CM=0 → R=0
If CC=1 & CM=m → R=1/m; max (m)=max value (CM)=8;
If CC=1 & CM=0 → R=0.1
Multimorbidity patterns were identified using the fuzzy c-means cluster analysis algorithm, which belongs to the family of soft clustering algorithms. The algorithm estimates c cluster centres (similar to k-means) but with fuzziness so that individuals may belong to more than one pattern. Through this technique, we obtained clusters of individuals and a membership matrix, which indicates the degree of participation of each subject in each cluster.
As a first step, and similarly to Violán et al,24 the PCAmix algorithm for categorical and continuous data (GS and R variables, respectively) was implemented to reduce and transform the dataset to all continuous data.25 To decide the number of retained dimensions, the Karlis-Saporta-Spinaki rule was used.26 Then, a soft clustering algorithm was applied to fuzzily distribute the population into a set of clusters, corresponding to the different multimorbidity patterns. We computed three validation indices to obtain the optimal number of clusters (K) and the optimal value of the fuzziness parameter (m): the partition coefficient whose optimal choice for coefficient is at the maximum and the Xie-Beni and the partition entropy validation indices, whose optimal indices are at the minimum.27 Considering the stochastic nature of the clusters, and the requirement of stable multimorbidity clusters, 100 independent clustering repetitions were applied to obtain the stable final solution.
To describe each identified cluster of individuals, the prevalence of CC and GS in each one was calculated. Observed/expected (O/E) ratios were calculated by dividing the prevalence of a given disease within a cluster by its prevalence in the overall population. The exclusivity of CC and GS, defined as the fraction of patients with the disease in the cluster over the total number of patients with the disease, was also calculated. A CC or a GS was considered to be relevant in a given cluster of individuals when its O/E ratio was >1 and its exclusivity was >25%.28–30 The statistical significant final solution ranged from 4 to 8 clusters. After clinical revision and discussion among the research team, four different clusters were considered to be consistent with the clinical observations as well as the objective of the clustering. There is currently no consensus in the literature on the criteria used to select the number of clusters or the O/E ratio cut-off point due to, in part, the novelty of the analysis.
Finally, sociodemographic and clinical variables were described for all patients assigned to each cluster. Analyses were performed using R V.3.6.0 and SPSS V.22.
Patient and public involvement
Since this was an observational study with variables and outcomes related to the healthcare process, this research was developed without patient involvement. Patients were not invited to comment on the study design and were not consulted to develop patient relevant outcomes or interpret the results.
Results
A total of 740 patients aged 65 years or older were included, with a mean age of 84.12 years (SD 7.01), a 53.24% of females and a mean Barthel Index of 65.07 (median 75). Sociodemographic and clinical variables are summarised in table 1. Almost all patients had two or more CC (98.65%; 95% CI 98.23% to 99.07%), with a median of 8 CC and 6 GS per patient. Nearly 70% had consulted a healthcare service in the 3 months prior to hospitalisation due to chronic disease exacerbation.
Table 1.
Sociodemographic and clinical variables of the studied cohort
| Sociodemographic and clinical variables | N | % | 95% CI | |
| Age | <70 | 33 | 4.46 | 3.19 to 6.20 |
| 70–74 | 48 | 6.49 | 4.93 to 8.50 | |
| 75–79 | 82 | 11.08 | 9.02 to 13.55 | |
| 80–84 | 181 | 24.46 | 21.50 to 27.68 | |
| 85–89 | 232 | 31.35 | 28.11 to 34.78 | |
| 90–94 | 134 | 18.11 | 15.50 to 21.05 | |
| ≥95 | 30 | 4.05 | 2.85 to 5.73 | |
| Sex | Female | 394 | 53.24 | 49.64 to 56.81 |
| Male | 346 | 46.76 | 43.19 to 50.36 | |
| Barthel Index | <20 | 90 | 12.16 | 10.00 to 14.71 |
| 20–35 | 76 | 10.27 | 8.28 to 12.67 | |
| 40–55 | 124 | 16.76 | 14.24 to 19.62 | |
| 60–95 | 294 | 39.73 | 36.27 to 43.30 | |
| 100 | 156 | 21.08 | 18.30 to 24.17 | |
| Age adjusted, updated Charlson Comorbidity Index | 2–5 | 148 | 20.00 | 17.28 to 23.03 |
| 6–8 | 411 | 55.54 | 51.94 to 59.08 | |
| 9–14 | 181 | 24.46 | 21.50 to 27.68 | |
| Household | With relatives/other people | 523 | 70.68 | 67.30 to 73.84 |
| Nursing home | 95 | 12.84 | 10.62 to 15.44 | |
| Alone | 122 | 16.49 | 13.99 to 19.33 | |
| Chronic disease exacerbation 3 months prior to admission |
No | 225 | 30.41 | 27.20 to 33.81 |
| Yes (total) | 515 | 69.59 | 66.19 to 72.80 | |
| Primary care | 342 | 46.22 | 42.65 to 49.82 | |
| Emergencies | 263 | 35.54 | 32.17 to 39.06 | |
| Hospital admission | 193 | 26.08 | 23.05 to 29.36 | |
| Outpatient care | 8 | 1.08 | 0.55 to 2.12 | |
| Home care | 14 | 1.89 | 1.13 to 3.15 | |
| Conditions requiring clinical management | 1 | 302 | 40.81 | 37.36 to 44.39 |
| 2 | 216 | 29.19 | 26.03 to 32.57 | |
| 3 | 106 | 14.32 | 11.98 to 17.03 | |
| 4 | 72 | 9.73 | 7.80 to 12.08 | |
| 5 | 28 | 3.78 | 2.63 to 5.41 | |
| 6 | 13 | 1.76 | 1.03 to 2.98 | |
| 7 | 2 | 0.27 | 0.07 to 0.98 | |
| 8 | 1 | 0.14 | 0.007 to 0.76 | |
| Destination at discharge | Home | 468 | 63.24 | 59.71 to 66.64 |
| Nursing home | 105 | 14.19 | 11.86 to 16.89 | |
| Another hospital | 101 | 13.65 | 11.36 to 16.31 | |
| Exitus | 66 | 8.92 | 7.07 to 11.19 | |
| Multimorbidity | No | 10 | 1.35 | 1.35 to 1.36 |
| Yes | 730 | 98.65 | 98.23 to 99.07 | |
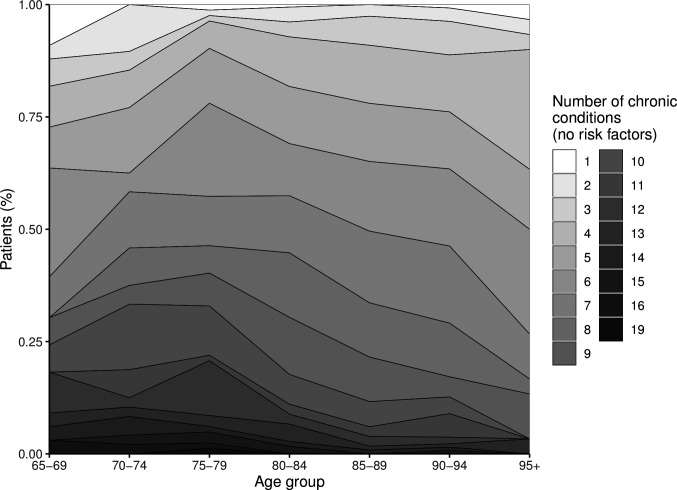
Figure 1 shows the distribution of the number of CC by age groups. The most frequent CC were hypertension (81.49%, 95% CI 78.53% to 84.12%) and heart failure (59.86%, 95% CI 56.29% to 63.34%) (see online supplemental table 2). Heart failure was also the main cause of hospitalisation (30.7% of patients had CM score=1), followed by chronic obstructive pulmonary disease (COPD) (20.7%) (online supplemental table 3).
Figure 1.
Distribution of the number of chronic conditions (excluding the following risk factors: hypertension, dyslipidaemia, obesity, osteoporosis and drug-related conditions) in relation to age groups.
There were some differences in CC between sexes, with females having more frequently heart failure, degenerative arthropathy, obesity, hip fracture, thyroid disease, asthma, osteoporosis, vertigo and non-schizophrenic mental disorders. Males, in turn, had more frequently COPD, gout, neoplasia, peripheral arteriopathy and ulcerative disease.
The most prevalent GS was polypharmacy (79.86%, 95% CI 76.82% to 82.60%), followed by frailty (61.76%, 95% CI 58.20% to 65.19%). Females had a significantly higher number of GS compared with males (Wilcoxon rank sum test, p<0.001), as well as a higher prevalence of depression/anxiety, chronic pain, constipation, frailty, urinary/faecal incontinence and immobility.
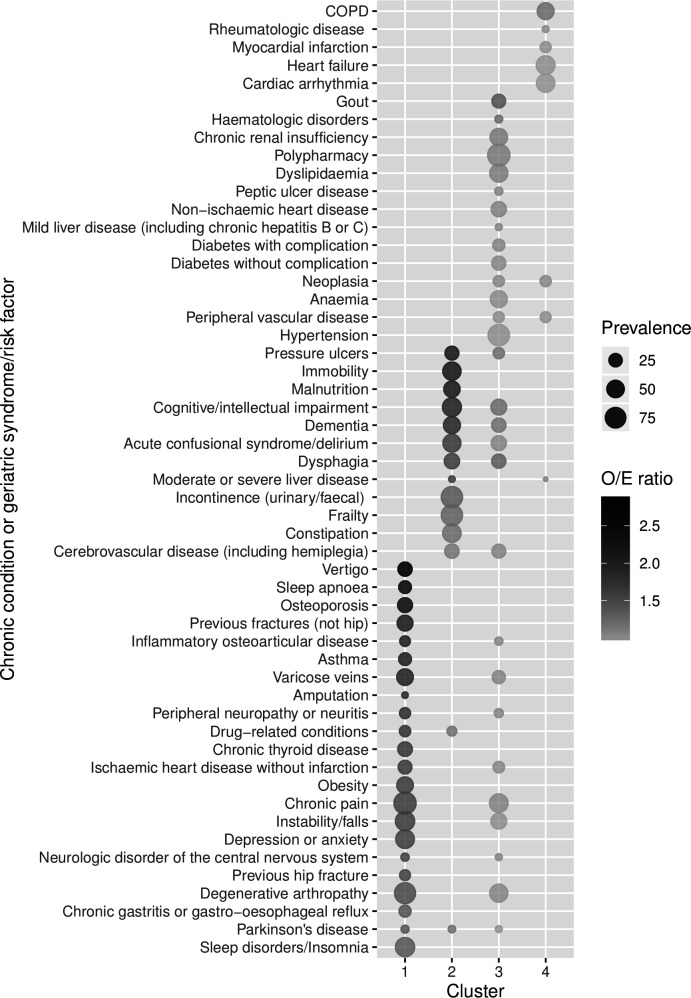
Four statistically and clinically significant multimorbidity clusters or patterns were identified in our study population. For all clusters, CC and GS with an observed/expected ratio >1 and exclusivity >25% are represented in figure 2 (see also online supplemental table 4) for all CC and GS). Sociodemographic and clinical characteristics of patients in each cluster are described in table 2.
Figure 2.
Observed/expected (O/E) ratio and prevalence of chronic conditions and geriatric syndromes/risk factors per multimorbidity cluster. Conditions with exclusivity >25% and O/E ratios >1 in each cluster are represented. Conditions are ordered by O/E ratio and from cluster 1 to 4. COPD, chronic obstructive pulmonary disease.
Table 2.
Sociodemographic and clinical variables of the multimorbidity clusters
| Osteoarticular | Psychogeriatric | Minor chronic disease | Cardiorespiratory | ||
| Number of patients included, n (%) | 132 (17.8) | 153 (20.7) | 179 (24.2) | 276 (37.3) | |
| Age at admission (year, mean±SD) | 84.03±6.48 | 84.51±7.25 | 83.94±7.19 | 84.06±7.03 | |
| Sex, n (%) | Male | 34 (25.7) | 66 (42.8) | 99 (55.5) | 147 (53.4) |
| Female | 98 (74.3) | 87 (57.2) | 80 (44.5) | 129 (46.6) | |
| Barthel Index (mean±SD) | 63.06±24.78 | 47.62±34.94 | 64.96±33.56 | 75.76±27.52 | |
| Total no chronic conditions (mean±SD) | 11.5±3.64 | 7.68±3.19 | 8.86±3.08 | 7.59±2.61 | |
| Total no geriatric syndromes/risk factors (mean±SD) |
7.76±2.07 | 8.16±2.82 | 6.4±3.32 | 4.42±2 | |
| Charlson Comorbidity Index, n (%) | 2–5 | 26 (19.9) | 24 (15.6) | 37 (20.4) | 61 (22.2) |
| 6–8 | 73 (55.1) | 89 (58.5) | 96 (53.8) | 153 (55.3) | |
| 9–14 | 33 (25.0) | 40 (25.9) | 46 (25.7) | 62 (22.6) | |
| Household, n (%) | With relatives/other people | 91 (68.7) | 103 (67.2) | 133 (74.5) | 196 (71.1) |
| Nursing home | 16 (11.8) | 28 (18.4) | 23 (12.8) | 28 (10.3) | |
| Alone | 26 (19.5) | 22 (14.3) | 23 (12.7) | 52 (18.7) | |
| Chronic disease exacerbation 3 months prior to the index admission, n (%) | No | 24 (18.3) | 46 (30.1) | 48 (27.0) | 106 (38.5) |
| Yes (total) | 108 (81.7) | 107 (69.9) | 130 (73.0) | 170 (61.5) | |
| Primary care | 83 (62.7) | 66 (43.3) | 92 (51.6) | 101 (36.5) | |
| Emergencies | 71 (53.6) | 40 (26.2) | 69 (38.5) | 83 (30.2) | |
| Hospital admission | 49 (37.1) | 46 (30.1) | 47 (26.1) | 51 (18.6) | |
| Outpatient care | 1 (0.7) | 0 (0.3) | 3 (1.4) | 4 (1.4) | |
| Home care | 2 (1.5) | 2 (1.1) | 3 (1.8) | 7 (2.6) | |
| Destination at discharge, n (%) | Home | 86 (65.3) | 83 (54.0) | 111 (62.0) | 188 (68.1) |
| Nursing home | 15 (11.1) | 33 (21.3) | 26 (14.5) | 32 (11.5) | |
| Another hospital | 15 (11.3) | 17 (11.3) | 26 (14.7) | 42 (15.3) | |
| Exitus | 16 (12.3) | 20 (13.3) | 16 (8.8) | 14 (4.9) |
The first cluster, named osteoarticular, included 132 patients (17.8%) having osteoporosis, fractures, inflammatory osteoarticular disease, chronic pain and degenerative arthropathy. Moreover, vertigo, sleep apnoea, asthma, depression/anxiety and sleep disorders were also over-represented. This cluster included patients with the highest number of both CC and GS. About three-quarters were female, and most of them (82%) accessed healthcare services 3 months prior to this admission.
Cluster 2, called psychogeriatric, had 152 patients (20.7%) and included mostly GS: pressure ulcers, immobility, malnutrition, cognitive impairment, dementia, incontinence and frailty. Patients in this group had a mean Barthel index lower than 50 and a high number of GS. Furthermore, nearly 20% of them were living in a nursing home and in-hospital mortality was about 13%.
Cluster 3, named minor chronic disease, had 179 (24.2%) patients, and represents a group of patients with a variety of conditions, such as hypertension, dyslipidaemia, anaemia, gout, chronic renal insufficiency, polypharmacy, non-ischaemic heart disease, and diverse GS. O/E ratios were close to 1 in most cases.
Finally, cluster 4, called cardiorespiratory, included 276 (37.3%) patients. The over-represented diagnoses were COPD, heart failure and cardiac arrhythmia, although the O/E ratios were very low. In this cluster, with the lowest number of CC and GS, and a Barthel index greater than 75, nearly 40% had no healthcare consultation for a chronic disease exacerbation in the previous 3 months. This group had the lowest in-hospital mortality (5%).
Discussion
This study aimed to identify multimorbidity patterns in patients aged 65 and above admitted to hospital because of an exacerbation of CC. The soft clustering technique used, together with clinical criteria, was able to identify four different multimorbidity patterns, named osteoarticular, psychogeriatric, cardiorespiratory and minor chronic disease, in a patient-centred approach taking into account the importance of each disease in hospital management. Remarkably, high chronic multimorbidity was found in all patients, regardless of the cluster. To the best of our knowledge, this is the only study published to date that has analysed multimorbidity patterns taking into account both CC (with their weight during CM) and GS in this type of patients. Hence, these identified patterns allow us to take a further step towards understanding the patients’ current or future healthcare needs.
Two very important aspects of multimorbidity patterns analysis are the purpose for designing such patterns and the target population, which clearly condition the obtained results or conclusions. For instance, our aim in defining multimorbidity patterns in this cohort was to identify profiles of patients with similar clinical needs during the index hospitalisation and even a similar short-term prognosis at that time. For this reason, the importance of their pathologies in the course of hospitalisation was also taken into account. Hence, the ones that tend to have a minimal impact on CM, such as risk factors like hypertension or dyslipidaemia did not have a leading role in the patterns.
All clusters contain coherent groups of conditions that are mostly pathophysiologically related. From the clinical point of view, these clusters resemble patient profiles that are intuitively perceived. Moreover, some descriptive variables such as sex, Barthel Index, mean number of CC or GS, chronic pathology exacerbations in previous months, or hospital mortality, are distributed in such a way that they may reinforce the distinction of these groups.
Coexistence of CC and GS was observed in all clusters except for the cardiorespiratory, reinforcing the need to consider other clinically relevant situations rather than only CC. In particular, the exclusivity and prevalence of GS such as immobility, malnutrition, cognitive impairment or dementia were considerable in the psychogeriatric cluster.
Interestingly, highly prevalent CC, such as heart failure and COPD, which also frequently involve CM, only showed remarkable exclusivity and O/E ratio in the cardiorespiratory pattern and were not over-represented elsewhere. This highlights the fact that even though some CC may not be over-represented in a cluster, they can have a high prevalence and therefore need to be properly addressed too.
With respect to the osteoarticular cluster, it displayed a pattern of female predominance, with many CC and GS, high healthcare needs in recent months due to their chronic pathology, and high in-hospital mortality. Thus, this profile would identify a group of patients with a high probability of decompensation and death.
Finally, the so-called minor chronic diseases cluster was not very well defined. It included some risk factors (hypertension, dyslipidaemia, polypharmacy) as well as some CC and GS. Thus, it would be possible that it does not represent a real cluster but either the set of cases that did not belong anywhere else.
Comparison with other studies
Given the type of patients under study and the methodological approach to identify multimorbidity patterns, there are few publications to directly compare our results to. Clerencia-Sierra et al 17 analysed multimorbidity patterns in hospitalised older patients. Their methodological and analytical approach was slightly different, and they did not take into account the weight of the diseases during the hospitalisation process; however, they found a similar percentage of multimorbidity (99.7%) and four patterns that partially coincide with those of our study: cardiovascular, induced-dependency, falls and osteoarticular.
Furthermore, several authors have published data on patterns identified from primary care electronic records in different age groups, with lists of non-comparable chronic problems and using different techniques (cluster analysis, exploratory factor analysis or latent class analysis).17 29–34 These results would not be directly comparable with our study, but all of them highlight the ability to identify association patterns of chronic diseases.
Strengths and limitations
The strengths of this study are the prospective design, ensuring data quality by thorough record keeping, the ascertainment of all CC and GS of the patient, as well as the use of a novel clustering technique. Soft clustering is a methodologically robust technique less susceptible to outliers in the data, choice of distance measure and the inclusion of inappropriate or irrelevant variables.24 Besides, our approach focuses beyond organ diseases by incorporating GS, and using a comprehensive list retrieved by the clinical team. Additionally, we have taken into account the relative importance of the different CC in the CM of the patient during hospitalisation, thus providing a better picture of the possible complexity and needs during hospitalisation.
Furthermore, our work is not only limited to the identification of possible patterns. We have validated them, in some way, by analysing some of the patients’ variables such as sex, number of CC, previous contacts with the health system, hospital mortality or need for a nursing home.
Nonetheless, our study presents some limitations that need to be considered. First, the identification of chronic pathologies does not exclusively follow a validated list of codes but either an adaptation of different ones, a fact that could hinder comparability with other studies on multimorbidity. Second, as this study is not longitudinal, the chronology in which CC or GS appear cannot be analysed. It is possible for a patient to evolve from one pattern to another throughout life, as some authors have already pointed out,35 and therefore, the results only show the present situation. However, given the purpose of the defined patterns, this would not in itself be a limitation.
From a clinical point of view, the lack of usage of standard scales or diagnostic criteria for determining all CC or GS could question the validity of this information. However, the study gathered the data as it was routinely registered in the different departments. Frailty should derive from a comprehensive assessment of the patient in a standardised way that still lacks of systematic implementation in the healthcare routine.36 Nevertheless, our multimorbidity study wanted to go a little further, also considering GS (frailty included), an unusual fact in the bibliography on clusters of multimorbidity in older patients in spite of its importance for decision making in the clinical practice.
The clinical conditions severity or other possible aggravating factors have neither been gathered. Nevertheless, the registration of a variable that takes into account the relevance of each CC during the care process acts, in some way, as a proxy of the importance of each disease in the index hospitalisation when dealing with patients admitted because of decompensation. Considering the purpose of defining the patterns in the whole study, and not knowing useful precedents in the consulted bibliography, the assignment made by the medical professional who attended the patient was an easy, simple measure, and shared by all professionals at the time of writing the clinical course.
Clinical implications
These patterns are not a picture of the community but of older patients in geriatric or internal medicine departments, which are generally in more need of health services and more complex CM. However, not all of these patients have the same requirements. In fact, one in five patients (the psychogeriatric cluster) caused a great burden to both the patient and their relatives while the patients in the most frequent cluster (cardiorespiratory), with lower dependency and less GS, seemed to have better immediate outcome. Therefore, it is possible that the therapeutic objectives should be different in these patients. More importantly, the ability to distinguish patients more objectively than with the mere clinical impression may allow the design of better processes, services or alternatives to conventional hospitalisation. Indeed, the identification of multimorbidity patterns in subsets of the population in order to detect underlying factors, understand their burden on patients and develop preventive strategies is considered a research priority. Finally, the development of clinical practice guidelines according to these patterns needs to be considered, although it may be difficult given the magnitude of the diseases comprised in each pattern.4 14 In addition, some patterns may include patients with an increased risk of potentially inappropriate prescription or adverse drug reactions. These aspects will be the object of future analyses.
Conclusions
In conclusion, in older patients admitted to hospital because of the exacerbation of chronic health problems, it is possible to define multimorbidity clusters or patterns using appropriate statistical techniques. These patterns seem clinically coherent and could be the basis to reorganise circuits, processes or healthcare models to tackle the increasing number of older, multimorbid patients.
Supplementary Material
Acknowledgments
The authors acknowledge the dedication and support of the entire MoPIM research group. We are grateful to Sandra Prados, Antonio Gimeno and Beatriz Poblador for their insightful comments, as well as to Blanca Rovira for her literature review as part of the final project of her medical degree at UAB, and to Celia Corral, for helping in tables and figures editing.
Footnotes
Twitter: @BareMarisa
Collaborators: MoPIM study group: Parc Taulí University Hospital: Marisa Baré (Clinical Epidemiology and Cancer Screening; REDISSEC), Susana Herranz (Acute care Geriatric Unit), Rosa Jordana (Department of Internal Medicine), Maria Queralt Gorgas (Pharmacy Department, REDISSEC), Sara Ortonobes (Pharmacy Department), Marina Lleal (Clinical Epidemiology and Cancer Screening); University Hospital of Vic: Pere Roura-Poch (Epidemiology Unit; REDISSEC), Daniel Sevilla, Núria Solà and Javier González (Pharmacy Department), Núria Molist, Mariona Espauella (Department of Geriatrics); Oscar Mascaró (Department of Internal Medicine); Hospital del Mar Medical Research Institute-IMIM: Elisabet de Jaime (Geriatrics Department), Olivia Ferrandez (Pharmacy Department), Maria Sala (Department of Epidemiology and Evaluation, REDISSEC), Miguel Ángel Márquez, Marta Arellano, Carlos Clemente and Olga Sabartés (Department of Geriatrics), Núria Carballo and Marta de Antonio (Pharmacy Department); Hospital de Galdakao: Rafael Estrada (Department of Internal Medicine), Maria Olatz Ibarra (Pharmacy Department); Complejo Hospitalario Universitario de Canarias: Candelaria Martin (Department of Internal Medicine), Gloria Julia Nazco (Pharmacy Department), Rubén Hernández (Department of Internal Medicine).
Contributors: MB conceived and supervised the study, discussed the results, wrote the first version of the manuscript and is responsible for the overall content as guarantor. SH, RJ, MA, RE and GJN participated in patient inclusion, data collection and discussion of the results and revised the manuscript. AR-L executed the analysis and interpretation of multimorbidity clusters. CV collaborated in the execution of the analyses and in the interpretation of the multimorbidity patterns. ML participated in the statistical and graphical analysis of the results, in the discussion of the results and the revision of several manuscript versions. PR-P collaborated in the questionnaires’ design, patient inclusion, data collection and discussion of results and revised the manuscript. All authors read and approved the final manuscript.
Funding: This work was supported by grants from Instituto de Salud Carlos III-FEDER, (PI15/00552) and Institut d'Investigació i Innovació Parc Taulí (I3PT) [CIR2017/0070], and by the Network for Research into Healthcare in Chronic Diseases, REDISSEC (RD16/0001/0002).
Disclaimer: These funding bodies had no role in the design of the study, nor in the collection, analysis and interpretation of data nor in writing the manuscript.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Contributor Information
the MoPIM study group:
Marisa Baré, Susana Herranz, Rosa Jordana, Maria Queralt Gorgas, Sara Ortonobes, Marina Lleal, Pere Roura-Poch, Daniel Sevilla, Núria Solà, Javier González, Núria Molist, Mariona Espauella, Oscar Mascaró, Elisabet de Jaime, Olivia Ferrandez, Maria Sala, Miguel Ángel Márquez, Marta Arellano, Carlos Clemente, Olga Sabartés, Núria Carballo, Marta de Antonio, Rafael Estrada, Maria Olatz Ibarra, Candelaria Martin, Gloria Julia Nazco, and Rubén Hernández
Data availability statement
Data are available on reasonable request. Data are available upon reasonable request to Dr. Marisa Baré (mbare@tauli.cat).
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study was approved by the clinical research ethics committees of each centre: Comité Ético de investigación Clínica del Parc Taulí, Comitè Ètic d'Investigació Clínica Osona per a la Recerca i Educació Sanitàries (FORES), Comité de Ètica de la Investigación con Medicamentos (CEIm)-Parc de Salut MAR, Comité Ético de Investigación Clínica de Euskadi, Comité de Ética de Investigación del Hospital Universitario de Canarias. No written informed consent was deemed necessary for this study.
References
- 1. Eurostat - Data Explorer. Available: https://appsso.eurostat.ec.europa.eu/nui/show.do?dataset=tps00200&lang=en [Accessed 21 Oct 2020].
- 2. OECD/EU . Health at a glance: Europe 2018 state of health in the EU cycle 2018.
- 3. Palladino R, Pennino F, Finbarr M, et al. Multimorbidity and health outcomes in older adults in ten European health systems, 2006-15. Health Aff 2019;38:613–23. 10.1377/hlthaff.2018.05273 [DOI] [PubMed] [Google Scholar]
- 4. The Academy of Medical Sciences . Multimorbidity: a priority for global health research, 2018. Available: https://acmedsci.ac.uk/file-download/82222577
- 5. Lugtenberg M, Burgers JS, Clancy C, et al. Current guidelines have limited applicability to patients with comorbid conditions: a systematic analysis of evidence-based guidelines. PLoS One 2011;6:e25987. 10.1371/journal.pone.0025987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Buffel du Vaure C, Dechartres A, Battin C, et al. Exclusion of patients with concomitant chronic conditions in ongoing randomised controlled trials targeting 10 common chronic conditions and registered at ClinicalTrials.gov: a systematic review of registration details. BMJ Open 2016;6:e012265. 10.1136/bmjopen-2016-012265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fortin M, Stewart M, Poitras M-E, et al. A systematic review of prevalence studies on multimorbidity: toward a more uniform methodology. Ann Fam Med 2012;10:142–51. 10.1370/afm.1337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Diederichs C, Berger K, Bartels DB. The measurement of multiple chronic diseases--a systematic review on existing multimorbidity indices. J Gerontol A Biol Sci Med Sci 2011;66:301–11. 10.1093/gerona/glq208 [DOI] [PubMed] [Google Scholar]
- 9. Roso-Llorach A, Violán C, Foguet-Boreu Q, et al. Comparative analysis of methods for identifying multimorbidity patterns: a study of 'real-world' data. BMJ Open 2018;8:e018986. 10.1136/bmjopen-2017-018986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Prados-Torres A, Calderón-Larrañaga A, Hancco-Saavedra J, et al. Multimorbidity patterns: a systematic review. J Clin Epidemiol 2014;67:254–66. 10.1016/j.jclinepi.2013.09.021 [DOI] [PubMed] [Google Scholar]
- 11. Jyoti Bora D, Kumar Gupta A. A comparative study between fuzzy clustering algorithm and hard clustering algorithm. Int J Comput Trends Technol 2014;10. [Google Scholar]
- 12. Lu F-P, Chang W-C, Wu S-C. Geriatric conditions, rather than multimorbidity, as predictors of disability and mortality among octogenarians: a population-based cohort study. Geriatr Gerontol Int 2016;16:345–51. 10.1111/ggi.12480 [DOI] [PubMed] [Google Scholar]
- 13. Heide vander, Snoeijs SP, Boerma WGW. How to strengthen patient-centredness in caring for people with multimorbidity in Europe? 2017. Available: http://europepmc.org/books/NBK464537 [PubMed]
- 14. Rijken M, Struckmann V, Heide VD. How to improve care for people with multimorbidity in Europe? 2016. Available: https://www.euro.who.int/en/about-us/partners/observatory/publications/policy-briefs-and-summaries/how-to-improve-care-for-people-with-multimorbidity-in-europe [PubMed]
- 15. NICE . Multimorbidity: clinical assessment and management NICE guideline, 2016. Available: www.nice.org.uk/guidance/ng56
- 16. Catalá-López F, Alonso-Arroyo A, Page MJ, et al. Mapping of global scientific research in comorbidity and multimorbidity: a cross-sectional analysis. PLoS One 2018;13:e0189091. 10.1371/journal.pone.0189091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Clerencia-Sierra M, Calderón-Larrañaga A, Martínez-Velilla N, et al. Multimorbidity patterns in hospitalized older patients: associations among chronic diseases and geriatric syndromes. PLoS One 2015;10:e0132909. 10.1371/journal.pone.0132909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Baré M, Herranz S, Jordana R, et al. Multimorbidity patterns in chronic older patients, potentially inappropriate prescribing and adverse drug reactions: protocol of the multicentre prospective cohort study MoPIM. BMJ Open 2020;10:e033322. 10.1136/bmjopen-2019-033322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shah S, Vanclay F, Cooper B. Improving the sensitivity of the Barthel index for stroke rehabilitation. J Clin Epidemiol 1989;42:703–9. 10.1016/0895-4356(89)90065-6 [DOI] [PubMed] [Google Scholar]
- 20. Salisbury C, Johnson L, Purdy S, et al. Epidemiology and impact of multimorbidity in primary care: a retrospective cohort study. Br J Gen Pract 2011;61:e12–21. 10.3399/bjgp11X548929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373–83. 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 22. Amblàs-Novellas J, Martori JC, Espaulella J, et al. Frail-VIG index: a concise frailty evaluation tool for rapid geriatric assessment. BMC Geriatr 2018;18:29. 10.1186/s12877-018-0718-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Quan H, Li B, Couris CM, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge Abstracts using data from 6 countries. Am J Epidemiol 2011;173:676–82. 10.1093/aje/kwq433 [DOI] [PubMed] [Google Scholar]
- 24. Violán C, Foguet-Boreu Q, Fernández-Bertolín S, et al. Soft clustering using real-world data for the identification of multimorbidity patterns in an elderly population: cross-sectional study in a Mediterranean population. BMJ Open 2019;9:e029594. 10.1136/bmjopen-2019-029594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chavent M, Kuentz-Simonet V, Labenne A. Multivariate analysis of mixed data: the R package PCAmixdata.. arXiv 2017. https://arxiv.org/abs/1411.4911v4 [Google Scholar]
- 26. Karlis D, Saporta G, Spinakis A. A simple rule for the selection of principal components. Commun Stat Theory Methods 2003;32:643–66. 10.1081/STA-120018556 [DOI] [Google Scholar]
- 27. Zhao Q. Cluster validity in clustering methods, 2012. Available: http://cs.joensuu.fi/sipu/pub/qinpei-thesis.pdf
- 28. Guisado-Clavero M, Roso-Llorach A, López-Jimenez T, et al. Multimorbidity patterns in the elderly: a prospective cohort study with cluster analysis. BMC Geriatr 2018;18:16. 10.1186/s12877-018-0705-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Marengoni A, Roso-Llorach A, Vetrano DL. Patterns of multimorbidity in a population-based cohort of older people: sociodemographic, lifestyle, clinical, and functional differences. J Gerontol A Biol Sci Med Sci 2019;75:798–805. [DOI] [PubMed] [Google Scholar]
- 30. Violán C, Roso-Llorach A, Foguet-Boreu Q, et al. Multimorbidity patterns with k-means nonhierarchical cluster analysis. BMC Fam Pract 2018;19:108. 10.1186/s12875-018-0790-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Juul-Larsen HG, Christensen LD, Bandholm T, et al. Patterns of Multimorbidity and Differences in Healthcare Utilization and Complexity Among Acutely Hospitalized Medical Patients (≥65 Years) - A Latent Class Approach. Clin Epidemiol 2020;12:245–59. 10.2147/CLEP.S226586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhu Y, Edwards D, Mant J, et al. Characteristics, service use and mortality of clusters of multimorbid patients in England: a population-based study. BMC Med 2020;18:78. 10.1186/s12916-020-01543-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nguyen QD, Wu C, Odden MC, et al. Multimorbidity patterns, frailty, and survival in community-dwelling older adults. J Gerontol A Biol Sci Med Sci 2019;74:1265–70. 10.1093/gerona/gly205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Poblador-Plou B, van den Akker M, Vos R, et al. Similar multimorbidity patterns in primary care patients from two European regions: results of a factor analysis. PLoS One 2014;9:e100375. 10.1371/journal.pone.0100375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Vetrano DL, Roso-Llorach A, Fernández S, et al. Twelve-year clinical trajectories of multimorbidity in a population of older adults. Nat Commun 2020;11:1–9. 10.1038/s41467-020-16780-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ward K, Reuben D. Comprehensive geriatric assessment. In: UpToDate, 2020. https://www.uptodate.com/contents/comprehensive-geriatric-assessment [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2021-049334supp001.pdf (120KB, pdf)
Data Availability Statement
Data are available on reasonable request. Data are available upon reasonable request to Dr. Marisa Baré (mbare@tauli.cat).