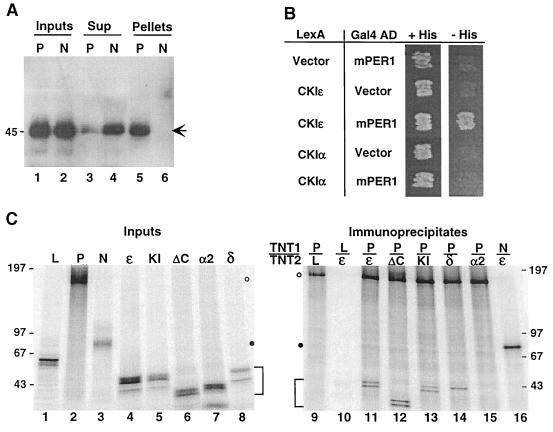
FIG. 1.
CKIɛ binds to mPER1 in vivo and in vitro. (A) Coimmunoprecipitation of mPER1 and endogenous CKIɛ. HEK 293 cells were transiently transfected with plasmids expressing either full-length Myc-mPER1 (P) or the amino-terminal fragment Myc-mPER1(1-485) (N). The PER proteins were immunoprecipitated from cell lysates with anti-Myc MAb 9E10; 20 μg of cell lysate protein (Inputs; lanes 1 and 2), the equivalent of 20 μg of the cell lysate supernatant following clarification and immunoprecipitation (Sup; lanes 3 and 4), and the immunoprecipitate pellet from 50 μg of cell lysate (Pellets; lanes 5 and 6) were analyzed by SDS-PAGE, followed by immunoblotting with anti-CKIɛ antibody UT31 (14). The arrow indicates the position of endogenous CKIɛ. (B) Specificity of the CKIɛ-mPER1 interaction assessed by two-hybrid assay. Yeast cotransformed with plasmids expressing the indicated proteins fused to either LexA or the Gal4 activation domain (AD) were grown on synthetic medium containing histidine (+His) or on medium containing 5 mM 3-aminotriazole and lacking histidine (−His) as previously described (37). Interaction between the indicated proteins was assessed by growth on −His plates. (C) Specificity of the CKIɛ-mPER1 interaction assessed by coimmunoprecipitation in vitro. In vitro-synthesized [35S]methionine-labeled proteins (Inputs; lanes 1 to 8) luciferase (L), Myc-mPER1 (P), truncated Myc-mPER1(1-485) (N), CKIɛ (ɛ), kinase-inactive CKIɛ(K38R) (KI), truncated CKIɛ(ΔC320) (ΔC), CKIα2 (α2), or CKIδ (δ) were mixed together (TNT1/TNT2) as indicated above lanes 9 to 16. Following a 30-min incubation, the protein mixtures were subjected to immunoprecipitation with anti-Myc MAb 9E10 and analyzed by SDS-PAGE (5 to 15% gel) (lanes 9 to 16). One-tenth of each of the in vitro synthesis reactions used for immunoprecipitation was loaded on the input gel (left). Data were collected and analyzed using a Molecular Dynamics PhosphorImager. Open and closed circles mark the positions of full-length and truncated Myc-mPER1, respectively; brackets mark positions of the various CKI proteins. Here and in subsequent figures, positions of the various protein molecular weight markers are indicated to the side of the gel, with the size of each marker expressed in kilodaltons.