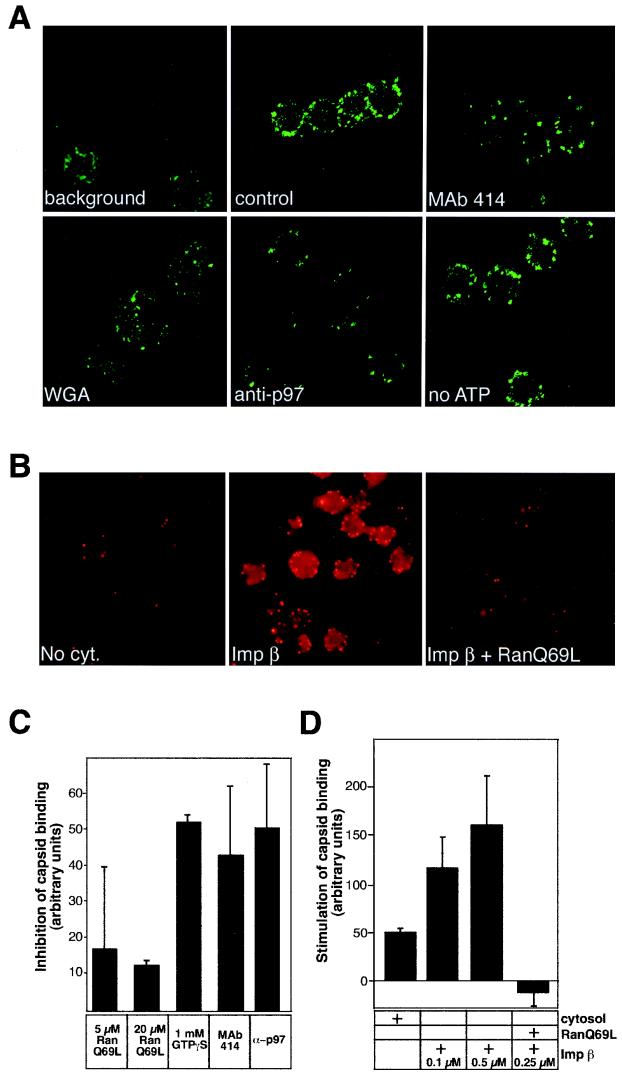
FIG. 4.
HSV-1 capsids bind to rat liver nuclei in vitro. (A) Confocal immunofluorescence microscopy of isolated HSV-1 capsids bound to purified rat liver nuclei in the presence of rat liver cytosol (control panel). The capsids were detected with RomV antibodies, which were generated against capsids isolated from virions. The background panel indicates a sample where capsids were omitted. Binding to nuclei pretreated with MAb 414, WGA, anti-importin β (anti-p97), or an ATP-depletion system (no ATP) is indicated in the other panels. (B) Conventional immunofluorescence microscopy of capsids binding to rat liver nuclei in the absence of cytosol (No cyt.), in the presence of 0.1 μM importin β (Imp β), or with 0.25 μM importin β and 10 μM RanQ69L (Imp β + RanQ69L). The capsids were detected as described for panel A. (C) Inhibition of capsid binding to nuclei pretreated with the indicated inhibitors in the presence of cytosol was quantitated as detailed in Materials and Methods. Inhibition of binding is shown relative to that of the control sample (without inhibitors) which represents the zero level in the graph. The mean values of at least triplicate samples with standard deviations are shown. (D) Stimulation of capsid binding to rat liver nuclei in vitro. Capsid binding to nuclei in the absence of cytosol (normalized to zero) or after addition of the components indicated on the right was quantitated as detailed in Materials and Methods. The mean values of at least triplicate samples with standard deviations are shown.