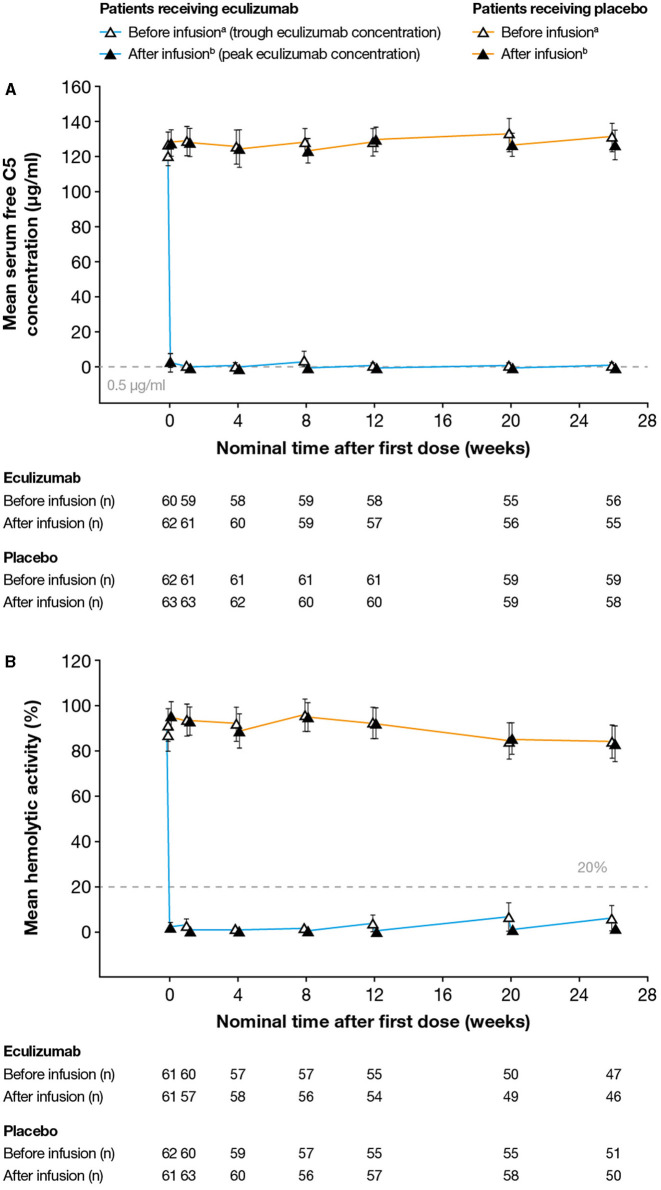
Figure 2.
Serum free C5 concentrations and complement-mediated hemolytic activity in serum during the study. (A) Mean (95% CI) serum free C5 concentrations. Free C5 concentrations below the lower limit of quantification (0.0274 μg/ml) were analyzed as 0.0137 μg/ml. Free C5 concentrations below 0.5 μg/ml (dashed line) indicate complete terminal complement inhibition. (B) Mean (95% CI) percent in vitro complement-mediated hemolytic activity of serum samples. Hemolysis values above 20% (dashed line) indicate incomplete inhibition of hemolysis. For both analyses, samples were taken before and after eculizumab infusion (i.e., at eculizumab serum trough and peak concentrations, respectively); samples taken before and after infusion in patients receiving placebo are shown for comparison. aSamples taken 5–90 min before infusion; bSamples taken 60 min after the completion of infusion; cDay 1. The numbers reported below the graphs are the numbers of patients for whom samples were tested at that timepoint. C5, complement protein 5; CI, confidence interval.