Figure 6. SynNotch CAR circuit T cells exhibit superior efficacy and persistence in vivo.
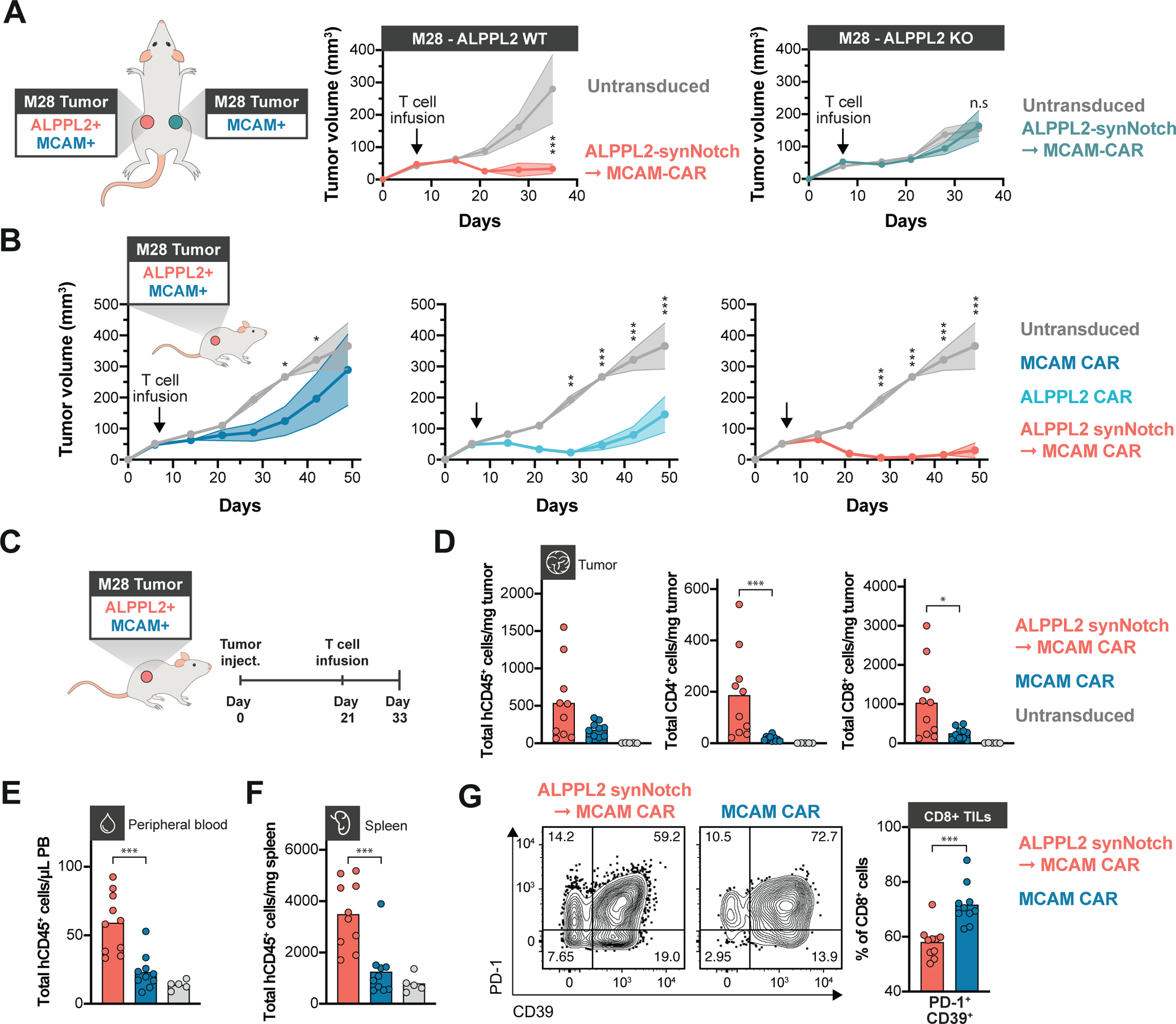
(A) NSG mice bearing contralateral subcutaneous tumors with ALPPL2 wild-type (WT) M28 tumors on the left side and with ALPPL2 knock-out (KO) M28 tumors on the right side received an adoptive transfer of a mix of CD4+ and CD8+ T cells engineered with an ALPPL2 synNotch→MCAM CAR circuit (n=5) or untransduced T cells (n=5). Tumor size was monitored over 35 days (representative of two independent experiments). (B) NSG mice bearing subcutaneous M28 tumors were injected i.v. with 1:1 ratio of CD4+:CD8+ T cells engineered with an ALPPL2 CAR (n=5), MCAM CAR (n=5), ALPPL2 synNotch regulating MCAM-CAR expression, or untransduced T cells (n=5). Tumor size was monitored over 49 days. (C) NSG mice bearing subcutaneous M28 tumors received an i.v. infusion of a 1:1 ratio of CD4+:CD8+ T cells engineered with either the MCAM CAR (n=10), the ALPPL2 synNotch→MCAM-CAR circuit (n=10), or untransduced T cells 21 days after tumor inoculation (n=5). Tumors, peripheral blood, and spleens were collected for phenotypic and numeric analysis of infused T cells 12 days post T cell infusion. (D) Number of tumor-infiltrating T cells (TILs) per mg tumor was determined by expression of human CD45 (hCD45) and CD4 or CD8. (E,F) Number of T cells per μL peripheral blood (E) and mg spleen tissue (F) was determined by expression of hCD45 (representative of two independent experiments). (G) Expression of PD-1 and CD39 in CD8+ TILs expressing a MCAM CAR either constitutively or through an ALPPL2 synNotch (representative of two independent experiments). Data are presented as mean±SEM. Statistics were calculated using either two-way ANOVA with Šidák’s (A) or Tukey’s post-hoc test (B), or unpaired Mann-Whitney’s t-test (D-G). *P ≤0.05; **P ≤0.01; ***P ≤0.001; n.s.; not significant.
