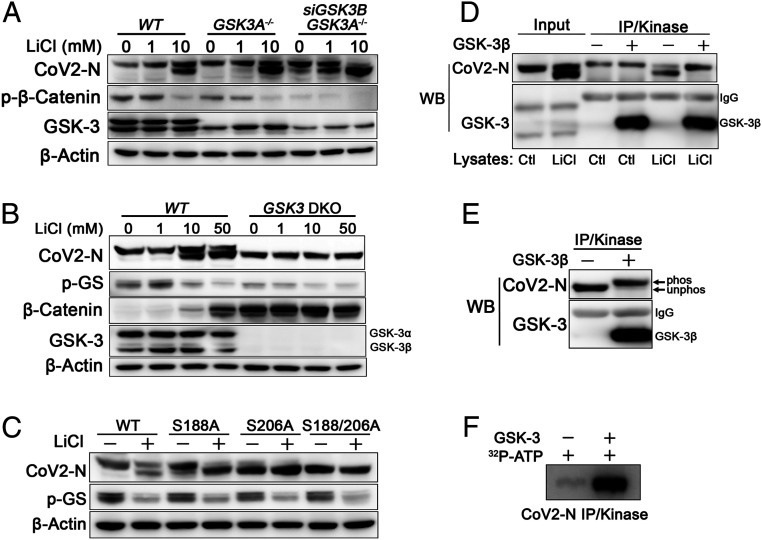
Fig. 2.
GSK3 is required for N phosphorylation. (A) Control 293T cells (WT), 293T cells with CRISPR/Cas9 KO of GSK3A (GSK3A−/−), and GSK3A−/− cells with siRNA knockdown of GSK3B (GSK3A−/−-;siGSK3B) were treated with LiCl at indicated concentrations and lysates were immunoblotted for N protein, phospho-β-catenin, GSK-3α/β, or β-actin. Combined loss of GSKA and GSK3B impairs phosphorylation of N and β-catenin and enhances sensitivity to LiCl. (B) GSK3B was deleted in GSK3A−/− cells using CRISPR (GSK3 DKO). N protein was expressed in both wild-type and DKO cells in the presence of increasing concentrations LiC for 18 h as above and immunoblotted for N protein, phospho-GS (pGS), total β-catenin, GSK-3α/β, and β-actin. N is not phosphorylated in DKO cells and mobility is not affected by LiCl treatment. Total β-catenin protein accumulates in absence of GSK-3 (DKO) (34) or upon inhibition with LiCl (35). (C) Serine-188 and serine-206 were mutated to alanine by site directed mutagenesis and single- and double-mutant N proteins were expressed in 293T cells in the presence of vehicle or 10 mM LiCl and immunoblotted for N protein, pGS, or β-actin. The double-mutant NS188A;S206A migrates similar to dephosphorylated wild-type N. Single mutants are more sensitive to LiCl. (D and E) N protein was immunoprecipitated from wild-type HEK293T cells treated with or without 10 mM LiCl for 18 h (indicated by “Ctl” or “LiCl” below each lane in D) or from GSK3 DKO cells (E). Immunoprecipitated N protein was added to an in vitro kinase reaction with recombinant GSK-3β. GSK-3β phosphorylates N from LiCl treated wild-type and DKO cells as indicated by slower electrophoretic mobility (“phos” in E). (F) N protein immunoprecipitated from DKO cells was added to an in vitro kinase reaction with recombinant GSK-3β as in E, except that γ-[32P]ATP was included and gels were fixed, dried, and exposed to X-ray film. WB, Western blot.