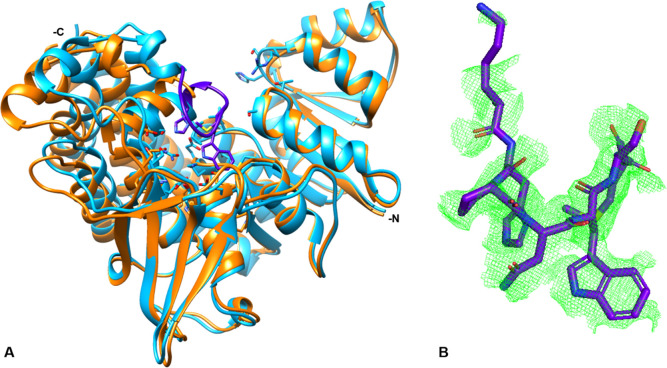
Figure 7.
The active site loop. (A) The AbIspC quaternary complex (blue) superimposed with apo AbIspC (PDB ID: 4ZN6, orange). The N- and C-termini are indicated. The active site loop (residues 216–223, violet) is closed over the active site in the quaternary complex. The conformation of both the N-terminal NADPH binding domain and the central catalytic domain are not greatly shifted by ligand binding. However, there is a distinct shift in the position of the four-helix bundle comprising the C-terminal α-helical domain. (B) Zoomed in view of the active site loop. The Fo-Fc electron density difference map, contoured at a radius of 2.8 Å around the loop region (residues 216–223), is shown in green mesh. Electron density can be observed for residues 216, and 220–223.