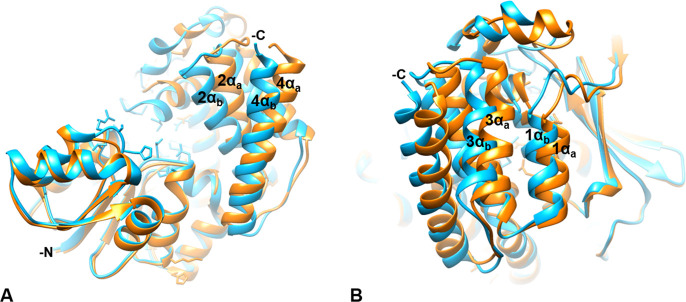
Figure 8.
The C-terminal α-helical domain. The AbIspC quaternary complex (blue) superimposed with apo AbIspC (PDB ID: 4ZN6, orange). The N- and C-termini are indicated where visible. The C-terminal α-helices adopt a more open conformation in the apo structure (1αa, 2αa, 3αa, 4αa), and a closed conformation in the quaternary complex (1αb, 2αb, 3αb, 4αb). (A) C-terminal α-helices 2 (residues 339–355) and 4 (residues 383–404) in the quaternary complex (2αb and 4αb) and in the apo conformation (2αa and 4αa). 2αb is shifted approximately 4.585 Å toward the active site relative to 2αa. 4αb is shifted approximately 5.880 Å toward the active site relative to 4αa. (B) C-terminal α-helices 1 (residues 325–335) and 3 (residues 361–374) in the quaternary complex (1αb and 3αb) and in the apo conformation (1αa and 3αa). 1αb is shifted approximately 2.163 Å toward the active site relative to 1αa. 3αb is shifted approximately 5.053 Å toward the active site relative to 3αa.