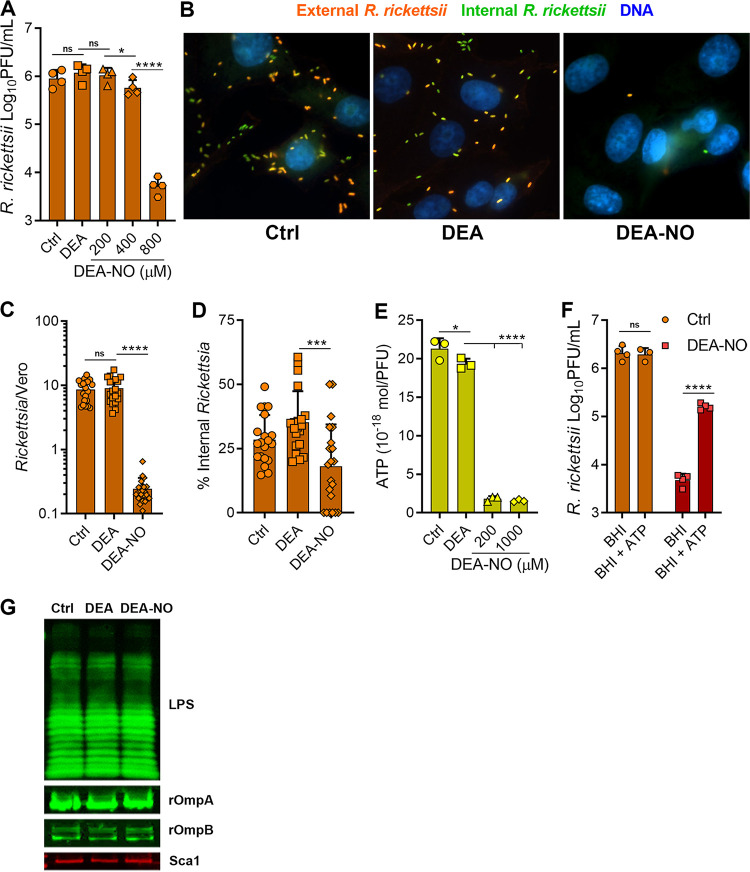
FIG 2.
NO decreases R. rickettsii adhesion to host cells. (A) R. rickettsii was challenged with DEA (800 μM) or DEA-NO in BHI medium for 10 min at 34°C in 5% CO2, and infectivity was determined by plaque counts (mean ± SD, n = 4). (B) Immunofluorescence microscopy of external and internal R. rickettsii cells treated with DEA or DEA-NO (images are representative of three or four independent experiments). (C and D) Quantification of images in panel B. The total number of R. rickettsii bacteria per cell was determined (C) and the proportion of internalized R. rickettsii was calculated (D) (five images were quantified from three or four independent experiments). (E) Nucleotides were extracted from R. rickettsii cells that had been challenged with DEA or DEA-NO for 10 min in BHI medium. Extracts were neutralized, and ATP content was determined with firefly luciferase and luminescence measurements (mean ± SD, n = 3). (F). R. rickettsii cells were challenged with or without 800 μM DEA-NO for 10 min in BHI medium. Samples were diluted into BHI medium or BHI medium supplemented with 1 mM ATP and incubated at 34°C in 5% CO2 for 30 min, and plaque assays determined infectivity (mean ± SD, n = 4). (G) R. rickettsii cells were challenged with DEA or DEA-NO (1 mM in BHI medium) for 10 min at 34°C and were analyzed by Western blotting. Antibodies against spotted fever rickettsial LPS (LPS), rOmpA, rOmpB, and Sca1 were used to detect R. rickettsii outer membrane antigens (representative blots, n = 2). Statistical analyses were performed using one-way ANOVA. *, P < 0.05; ***, P < 0.001; ****, P <0.0001; ns, not significant.