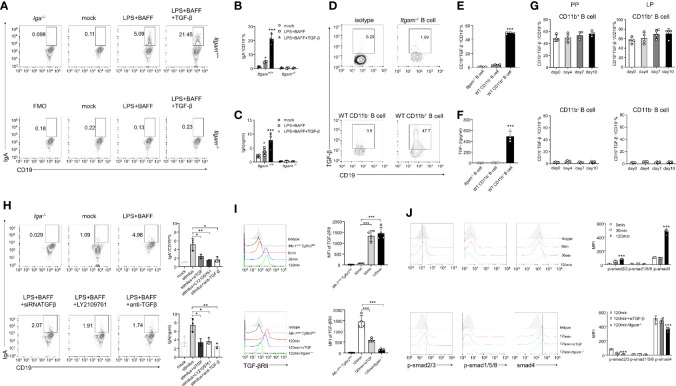
Figure 5.
CD11b-deficient altering of Smad signaling in B cells resulted in reduced TGF-β production and IgA isotype switching. (A) Purified PP-derived Itgam+/+ and Itgam−/− B cells of WT mice or Iga-/- mice were stimulated with LPS, BAFF, and TGF-β for 72 h Subsequently, IgA+ B cells were analyzed using flow cytometry (The Iga-/- group was shown as a negative control). (B) The frequency of IgA-expressing cells is displayed. (C) The culture supernatant was harvested to determine the production of IgA via ELISA. (D) The population of TGF-β+ B cells in the PPs from DSS-treated mice was analyzed using flow cytometry. (E) The frequency of TGF-β-expressing cells is displayed. (F) Expression of TGF-β in mouse serum was measured using ELISA. (G) Expression of TGF-β in CD11b+ B cells and CD11b− B cells in the PPs and colorectal LP at days 0, 4, 7, and 10 after treatment with DSS was detected using flow cytometry. The frequency and absolute number of IgA+ cells are presented. (H) The splenic B cells of mice were purified and transfected with small interfering RNA targeted for TGF-β (siTGF, other groups were transfected with the negative control siRNA for contrast). All groups were subsequently stimulated by LPS, BAFF, and TGF-β for 72 h in vitro. LY2109761 (5 µM) and anti-TGF-β (4 µg/mL) were used to inhibit TGF-β or TGF-β receptor, and the percentage of IgA+ B cells was measured using flow cytometry, and the IgA level in culture supernatant was measured using ELISA. The splenic B cells from the PP of WT mice, Mb-1cre/-Tgfbr2fl/fl , and Itgam−/− mice were purified and subsequently stimulated by LPS, TGF-β, or anti-TGF-β (αTGF, 4 µg/mL) for 0, 30, and 120 min. Expression of TGF-βRII (I) and the phosphorylation levels of Smad1/5/8 and Smad2/3 (J) were analyzed using flow cytometry. *P < 0.05; **P < 0.01; ***P < 0.001. Data are expressed as the mean of three independent experiments.