Box 1, Figure I. Septin Domains and Assembly.
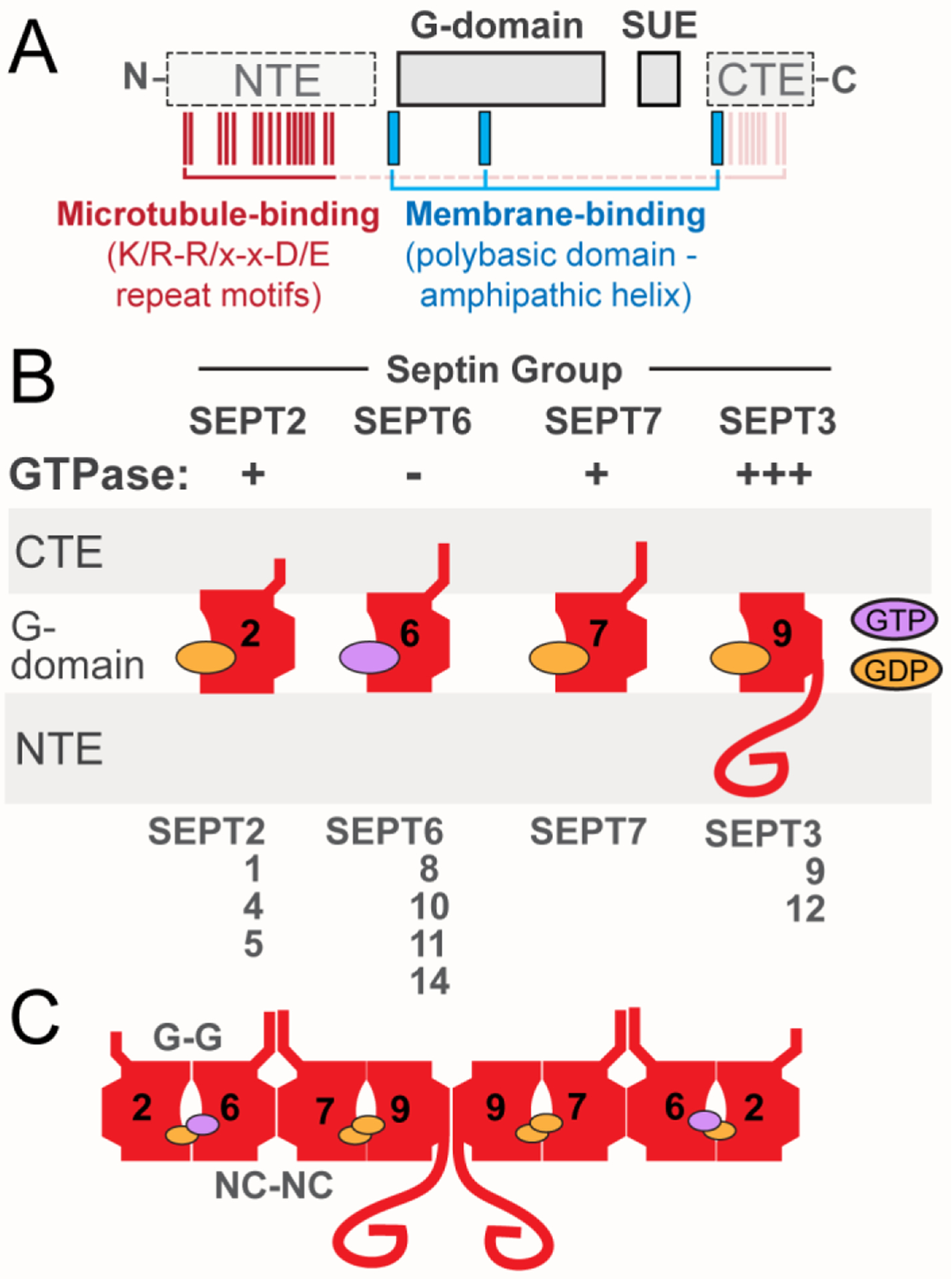
(A) Schematic shows the main domains of septins, which include the highly conserved G-domain and SUE, and the variable NTE and CTE. The positions of the membrane-binding polybasic domains and amphipathic helices (blue), and the microtubule-binding repeat motifs (K/R-R/x-x-D/E; red) are also depicted. Microtubule-binding motifs are present in the NTE of SEPT9, but similar motifs may mediate the interaction of the CTEs of SEPT6 and SEPT7 with microtubules. (B) Schematic of the position and orientation of the major domains of septins and the GTP/GDP nucleotide. GTPase activity is denoted as minus (−) for septins of the SEPT6 group, which do not hydrolyze GTP, and plus (+) for septins with slower GTPase such as SEPT2 and SEPT7. SEPT9, which is depicted as the ubiquitously expressed septin paralog of the SEPT3 group, has faster rates (+++) of GTP hydrolysis than SEPT2 and SEPT7. (C) Schematic representation of the prototypical SEPT2/6/7/9 hetero-octamer, which consists of septin paralogs interacting with one another through two different bindings interfaces, which involve the GTP-binding pockets (G-G interface) and the N- and C-termini of the GTP-binding domains (NC-NC interface).
