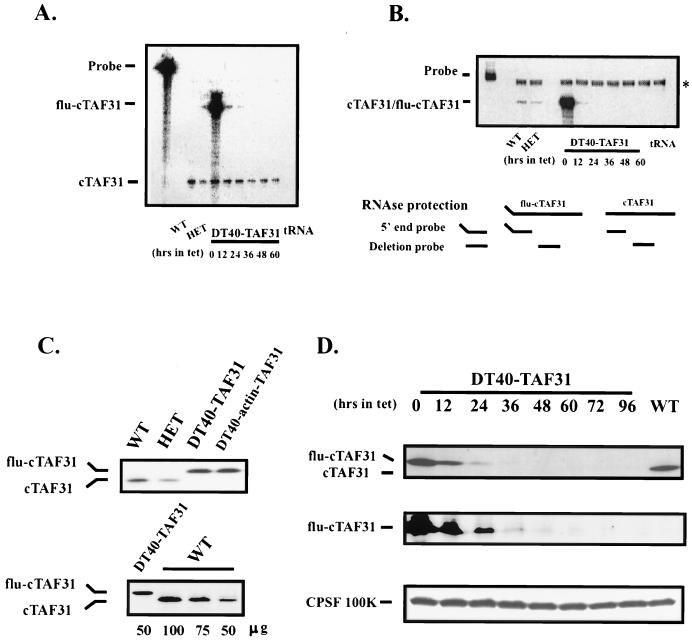
FIG. 3.
Expression of cTAFII31 in DT40-TAF31 cells. (A) RNase protection assay using a 5′-end probe. Ten micrograms of total RNAs from DT40 cells (WT), heterozygous cells (HET), and DT40-TAF31 cells incubated in the presence of Tet (1 μg/ml) for the indicated times was analyzed by RNase protection. The Flu tag sequence accounts for the size difference between endogenous and exogenous expression. tRNA was used as a negative control. (B) RNase protection assay using a deletion probe. The probe corresponds to part of the region deleted during gene targeting events (diagrammed below). Assay conditions are as above. The position of a background band, which is also present in the tRNA control lane, is indicated by an asterisk. (C) Western blot analysis using an affinity-purified polyclonal anti-cTAFII31 antibody. (Top) Thirty micrograms of total cellular proteins from wild-type, heterozygous, DT40-TAF31, and DT40-actin-TAF31 cells that was fractionated on an SDS–10% PAGE gel, transferred to a nitrocellulose membrane, and probed with an anti-chicken TAFII31 polyclonal antibody. Positions of flu-cTAFII31 and cTAFII31 are shown. (Bottom) The indicated amounts of total cell proteins from wild-type or DT40-TAF31 cells were analyzed as shown in the top panel. (D) Western blot of time course of Tet-induced depletion. DT40-TAF31 cells were incubated in the presence of Tet (1 μg/ml) for the indicated times before harvest. Lysates prepared from these cells were analyzed for Tet-repressible expression of flu-cTAFII31 using the above anti-cTAFII31 antibody (upper panel), a monoclonal antibody against the Flu tag (middle panel), and a polyclonal antibody against CPSF-100 (lower panel). Wild-type cell lysates (WT) were analyzed for comparison.