Abstract
As an indispensable component of the extracellular matrix, perlecan (Pln) plays an essential role in cartilaginous tissue function. Although there exist studies suggesting that Pln expressed by cartilaginous tissues is critical for chondrogenesis, few papers have discussed the potential impact Pln may have on cartilage regeneration. In this review, we delineate Pln structure, biomechanical properties, and interactive ligands—which together contribute to the effect Pln has on cartilaginous tissue development. We also review how the signaling pathways of Pln affect cartilage development and scrutinize the potential application of Pln to divisions of cartilage regeneration, spanning vascularization, stem cell differentiation, and biomaterial improvement. The aim of this review is to deepen our understanding of the spatial and temporal interactions that occur between Pln and cartilaginous tissue and ultimately apply Pln in scaffold design to improve cell-based cartilage engineering and regeneration.
Keywords: Perlecan, Cartilaginous tissues, Stem cell, Tissue regeneration, Pericellular matrix, Extracellular matrix
Graphical Abstract
Perlecan’s potential application in cartilage regeneration.
1. Introduction
Lacking blood vessels, nerves, and lymphatics, articular cartilage is a unique tissue with a limitation in intrinsic repair and healing. Articular cartilage, meniscus, and nucleus pulposus (NP) are cartilaginous tissues with unique properties but they share similar functionalities [1]. Extracellular matrix (ECM) secreted by chondrocytes is composed of type II collagen (COL II), glycosaminoglycan (GAG), elastin fibers, and laminin. Structurally, from the inside out, the ECM surrounding chondrocytes is divided into three layers: the pericellular matrix (PCM), the territorial matrix, and the interterritorial matrix [2].
Basement membrane (BM) is a ubiquitous structure of the ECM, which separates cells from and attaches them to the interstitial matrix [3] and exists in various tissues of the human body [4]. BM is an essential structure for maintaining various roles in tissue function, including but not limited to signal transduction, acting as a mechanical barrier, and tissue organization [5,6]. In chondrocytes, the distribution of BM components, including laminin, COL IV, nidogen, and perlecan (Pln), varies with age; initially widely distributed in the territorial and interterritorial matrix of mouse cartilage, BM components become primarily localized to the narrow PCM of the chondrocyte during maturation [7]. As such, during cartilage development, the PCM may constitute an integral ingredient of the dynamically modulated chondrocyte BM [7].
Perlecan (Pln), encoded by a heparan sulfate (HS) proteoglycan 2 (HSPG2) gene sited on the telomeric region of human chromosome 1 or of mouse chromosome 4, is one of the most abundant HSPGs in not only the BM but also many other tissues lacking typical BM structure and blood vessels such as the cartilage, meniscus, and intervertebral disc (IVD) [8–10]. There exists strong evidence that Pln plays a critical role in brain, bone, heart, and cartilage development [11,12]. Mutations in the HSPG2 gene result in two classes of skeletal disorders: Schwartz-Jampel syndrome, a mild disorder with increased risk of bone/cartilage loss, and Silverman-Handmaker type, a severe neonatal lethal dyssegmental dysplasia [13]. Pln deficiency is also a risk factor for osteoporosis [14].
Chondrocytes are enveloped by PCM, a narrow tissue region that, together with the enclosed chondrocytes, has been termed the “chondron”; the PCM, as a functional equivalent of BM, is distinguished from ECM in regard to its biochemistry, structure, and biomechanics [7]. In experimental settings where the HS chain of Pln is digested with heparinase III, no subsequent effect is observed on the elastic modulus of ECM, while the overall elastic modulus of both the general PCM and its interior regions increases [15]. This finding indicates that, besides COL VI, Pln can be considered as a defining factor exclusively localized to the PCM, which has also been found to have a significant effect on the structural integrity and biomechanical properties of PCM [15].
Given that HSPG2 gene regulation, structure, and function in chondrogenesis have been well summarized [16–19], in this review, we will outline present knowledge of the biomechanical and biochemical roles Pln plays in cartilage and, specifically, the effects Pln has on the development of articular cartilage, meniscus, and spinal tissues. Considering that Pln was also found to be substituted with both HS and/or chondroitin sulfate (CS) [20,21], this review will further discuss the role of the Pln CS chain in cartilage regeneration and reconstruction. Finally, this review will elucidate Pln’s role within the signaling pathways involved in cartilaginous tissue development, which might provide insights in favor of cartilage regeneration and reconstruction.
2. Pln structures
Pln is a secreted HSPG that was first isolated in 1980 from Engelbreth–Holm–Swarm tumor cells [22]. The name Pln is derived from the pearls-on-a-string-like appearance as shown by rotary shadowing electron microscopy. Human Pln has a 467 kDa protein core and five well-defined domains (I through V) of which domain I is exclusive to the proteoglycan (PG), while domains II-V share homology with various biological components [23–25]. (Figure 1) (Table 1)
Figure 1.
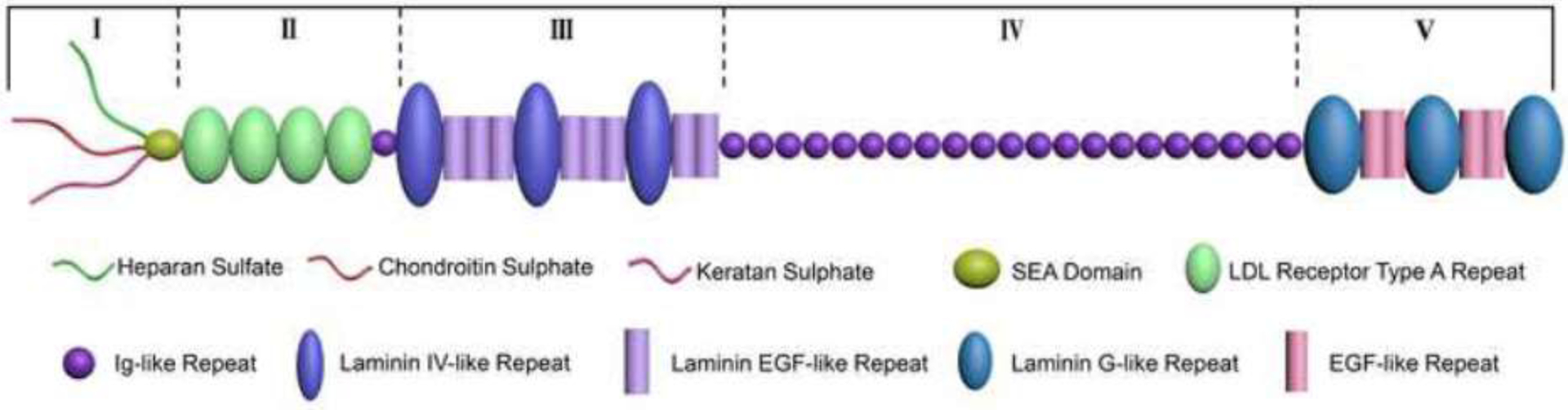
Schematic illustration of the multidomain structure of human Pln. The core protein of Pln consists of five domains I–V [194]. Domain I is made up of a sperm, enterokinase and agrin (SEA) fold, preceded by three GAG attachment sites. Domain II contains four low-density lipoprotein (LDL) receptor motifs and one isolated immunoglobulin-like (Ig) fold. Domain III is made up of a combination of laminin epidermal growth factor (EGF) and laminin IV type A domains that are connected by disulfide linkages in the laminin EGF domains to form an inflexible rod-like structure. In humans, domain IV consists of 21 repeating Ig C2-type modules that are sequentially linked together. Domain V comprises four EGF motifs, three laminin G, and a fourth variable GAG attachment site.
Table 1.
The protein domains of perlecan and their functions through interactive ligands.
| Domain | Interacting partner(s) | Potential function | References |
|---|---|---|---|
| I (HS) | Activin A, Ang-3, BMP-2, FBN1, FGF-2/18, GM-CSF, HGF, VEGF, WARP, PRELP, LN, PDGF | Angiogenesis; Cell adhesion/motility; Chondrogenesis; GF delivery/activity; ECM anchoring/assembly/stabilization | [27,30,31,48,141,195–198] |
| II | CTGF, FBN1, LDL/VLDL, Wnt/Ca2+ | BM anchoring and biogenesis of microfibrils | [27–30] |
| III | FGF-7/18, PDGF, WARP | Angiogenesis; Cartilage structure assembly; ECM assembly/stabilization; GF delivery | [31–33,199] |
| IV | COL IV, FBLN2, FN, Heparin, LN, NID1/2, PDGF | Angiogenesis; BM stabilization; GF reservoir | [27,200,201] |
| V | α2β1 Integrin, DG, ECM1, FBLN2, FGF-7, Heparin, NID1, PGRN, PRELP | Angiogenesis; BM assembly/stabilization; Cell adhesion and motility; Proliferation | [27,32,195,202,203] |
Abbreviation: Ang-3: angiopoietin-3; BM: basement membrane; BMP-2: bone morphogenetic protein-2; DG: dystroglycan; ECM: extracellular matrix; ECM1: extracellular matrix protein 1; FBLN2: fibulin-2; FBN1: fibrillin-1; FGF: fibroblast growth factor; FN: fibronectin; GF: growth factor; GM-CSF: granulocyte-macrophage colony-stimulating factor; HGF: hepatocyte growth factor; LDL: low-density lipoprotein; LN: laminin; NID: nidogen; PDGF: platelet-derived growth factor; PGRN: progranulin; PRELP: proline/arginine-rich end leucine-rich repeat protein; VEGF: vascular endothelial growth factor; VLDL: very low-density lipoprotein; WARP: von Willebrand factor A domain related protein
2.1. Domain-specific structures and interactions
Human Pln domain-I (PlnDI), the domain exclusive to Pln, contains three potential binding sites for HS and a sea urchin sperm protein, enterokinase, agrin (SEA) module [23,24,26,27]. PlnDI is capable of interacting with vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF), bone morphogenetic factor-2 (BMP-2), platelet-derived growth factor (PDGF), hepatic growth factor, granulocyte-macrophage colony-stimulating factor, and angiopoietin-3 through its HS side chains [27]. Through these interactions, PlnDI activates various intracellular signaling pathways. Furthermore, PlnDI also interacts with components, such as sonic hedgehog, von Willebrand factor A domain related protein (WARP), proline and arginine rich leucine rich repeat protein, laminin, COL IV, V, VI, and Xl, nidogen-1, fibronectin, thrombospondin-1, fibrillin-1, interleukin 2 (IL-2), IL-8, and activin A to stabilize the ECM and BM [27].
Pln domain II (PlnDII) contains four copies of internal repeats highly homologous to the low-density lipoprotein (LDL)-binding region of the LDL receptor [23]. Following the LDL receptor-like domain, there exists a short segment between domains II and III that is homologous to the repeats of immunoglobulin (Ig) that are also found in domain IV [23]. This Ig-like repeat can modulate calcium binding and mediate Wnt/calcium signaling [27]. In addition, PlnDII also interacts with very low-density lipoprotein, connective tissue growth factor/hypertrophic chondrocyte-specific gene product 24 (CTGF/Hcs24), and fibrillin-1 [28–30].
Pln domain III (PlnDIII) resembles the short arm of laminin chains [25]. It contains four internal cysteine-rich repeat subdomains that are separated by three laminin-like globular domains [23–25]. PlnDIII interacts with FGF-7/18, FGF-binding protein, and WARP [26,31–33].
Pln domain IV (PlnDIV) is the largest domain. Human PlnDIV contains 21 consecutive Ig-like repeats similar to those found in the neural cell adhesion molecule, whereas mouse PlnDIV only contains 14 consecutive Ig-like repeats [23,34,35], which decreases the core protein to 400 kDa [36]. This finding suggests that interspecies differences exist in the number of consecutive Ig-like subdomains. Additionally, the 14th Ig-like repeat of human Pln contains a potential GAG chain attachment sequence [24]. PlnDIV plays a crucial role not only in cartilage development through triggering chondrocyte condensation [37] but also in BM stabilization through interactions with fibronectin, nidogen-1/2, COL IV, fibulin-2, and PDGF [27].
Pln domain V (PlnDV) contains three laminin-like globular domains with two epidermal growth factors (EGF)-like domains among them; like PlnDIII, the three globular domains are similar to A chains of laminin [24,25]. Besides interacting with nidogen-1, FGF-7, fibulin-2, α-dystroglycan, progranulin, β1-integrin, ECM-1, acetylcholinesterase, and COL VI [27], PlnDV itself independently inhibits endothelial cell motility, tube formation, and blood vessel growth in angiogenesis assays [38].
The different domains each play a respective role in chondrogenesis. However, PlnDI and its binding GAG chains are often considered the most important as they provide the initial signal to activate chondrogenesis [39]. C3H10T1/2 cells, the mesenchymal fibroblast cell line, are able to enter chondrogenic differentiation when plated on the ECM protein Pln; however, these cellular Pln-stimulated nodules fail to express the chondrogenic maturation and subsequent terminal differentiation markers unless they are treated with BMP-2 [40]. This regulatory effect can be explained through two hypothesized mechanisms: (1) GAG chains could bind directly to cell surface receptors and stimulate cartilage differentiation through activating intracellular signaling pathways [39,41] and (2) GAG-bearing PGs could be able to serve as cell adhesion inhibitors that prevent cells from forming focal adhesions [39,42].
2.2. Tissue source-dependent structural variation
An interesting study comparing the effects of ECM from different cell sources on cartilage regeneration concludes that ECM differs in its function depending on the cell sources used [43]. Similarly, Pln from different cell sources is speculated to play distinctive roles in cartilage regeneration. Specialized cell-derived Pln has distinctive HS chain substructures, which may lead to differences in its ability to bind to growth factors [44]. Specifically, Pln derived from smooth muscle cells, IVD, and chondrocytes has both HS and CS chains, whereas Pln from endothelial cells contains exclusively HS chains [45,46]. Similarly, Pln from smooth muscle cells binds both FGF-1 and FGF-2 through its HS chains and promotes FGF-2 signaling but not FGF-1 signaling, while Pln from endothelial cells binds both FGF-1 and FGF-2 and promotes the signaling of both [45]. During BaF-32 cell (an IL-3 dependent and HSPG deficient myeloid B cell line [47]) proliferation, chondrocyte-derived Pln binds both FGF-2 and FGF-18 and forms HS chain-dependent ternary complexes with FGF-18 and FGF receptor 3 (FGFR3) [48]. Pln from endothelial cells can support BaF-32 cell proliferation with both FGF-1 and FGF-9, while Pln from chondrocytes is only responsive with FGF-9 [49]. Based on this differential bioactivity, it would be worthwhile to investigate which cell source would derive the most suitable version of Pln for use in cartilage regeneration, as there is no relevant research on the topic at present. The efficacy of adult stem cells in lineage-specific differentiation is greatly affected by the type of resident tissue from which they are isolated [50,51], which may expand our knowledge base.
3. Pln expression during development of cartilaginous tissues
As an essential component in these cartilaginous tissues [1], Pln plays a crucial role in developmental regulation.
3.1. Pln expression in the development of cartilage
When examining developing mouse embryos, approximately 40% of Pln-null mice at embryonic day 10.5 (E10.5) die as a result of defective cephalic development and about 60% of Pln-null mice die just after birth due to respiratory failure [52]. Only 6% of Pln-null mice develop both chondrodysplasia and exencephaly [52], which resembles Schwartz-Jampel syndrome [53]. However, loss of Pln does not affect early embryonic development, as results from E5.5 and E9.5 show that lack of Pln does not decrease the mouse embryo implantation rate [54]. Immunolocalization of Pln is first detected in angiogenic tissue at E10.5, earlier than in cartilaginous tissues; then from E11 to E13, Pln is abundantly expressed in developing cartilage undergoing endochondral ossification [55]. Genetic analysis indicates that, in normal limb bud cartilage, HSPG2 mRNA is first detected at E13.0 and shows weak expression in hypertrophic chondrocytes at E14.0; in condylar cartilage, HSPG2 mRNA is first detected in newly formed cartilage at E15.0 and declines in expression in the hypertrophic chondrocyte region [56]. Meanwhile, Pln immunoreactivity is evident throughout the tibial cartilage, indicating that these cells which develop into mature chondrocytes actively synthesize Pln, while hypertrophic chondrocytes downregulate Pln synthesis [56]. Loss of Pln reduces proliferative activity in E14.5 cartilage and aggrecan quantity in E16.5 cartilage, but has little effect on COL II [52]. Additional studies looking at human specimens have found that PlnDIII and V are expressed in cartilage anlagen and zones of chondral ossification beginning at gestational week (GW) 8 [57]. After GW 12, Pln becomes strongly localized to developing cartilaginous tissues in humans [49].
As Pln supports cartilage maturation [55] and chondrocyte differentiation [58], Pln expression persists from postnatal, to juvenile, adolescent, and adult cartilage in human samples [49]. During this time, Pln may be present in forms possessing HS chains, CS chains, or both. During chondrocyte differentiation, Pln deposition is increased and the sulfation pattern of its CS chains changes depending on the different cell zones of the growth plate [59]. When Pln distribution is measured in aging cartilage—ranging from newborn to eight-year-old sheep—a significant age-dependent decline in Pln levels is observed in hyaline and growth plate cartilage [60].
3.2. Pln expression in the development of meniscus
As seen in articular cartilage, Pln is also expressed during the development of the meniscus, a wedge-shaped fibrous cartilage located in the knee joint. While immature meniscus is full of blood vessels and rich in blood supply, later in development, this blood supply gradually decreases [61]. In adulthood, the blood supply of the meniscus only exists in the peripheral 10%−30% [1]. Pln distribution in the meniscus may be partly related to its special blood supply structure, as Pln is a core component of vascular BM [47]. In a study examining the meniscus of merino sheep (from newborn to 19-month-old), Pln is found expressed in the inner third of the meniscus, co-localized with COL II and aggrecan [62]. A similar study based on the ovine lateral meniscus of merino sheep demonstrates that Pln exists in the inner and middle meniscal zones of 7- to 19-month-old samples but decreases in 7- to 10-year-old samples [63]. Interestingly, atomic force microscope (AFM) shows that micromechanical properties of the meniscus Pln-labeled PCM and ECM display regional variations; the elastic modulus of the Pln-labeled PCM in the outer region was significantly higher than the inner region, and ECM moduli were constantly higher than region-matched PCM sites in both the outer and inner regions [9].
3.3. Pln expression in the development of IVD
As a component of PCM, Pln is found in various embryonic and adult tissues, including the spinal tissues of animals and humans [52,55,58,64]. In developing mouse embryos, the initial accumulation of Pln can be detected in the IVD and basal lamina of surrounding tissues at E13.5, and high levels of Pln begin to accumulate in the cartilage primordium of these regions at E15.5 [58]. Similar research also demonstrates that Pln expression in the cartilage primordium of the vertebral bodies begins at E10.5 and becomes prominent by E13; by E14, Pln deposition is rich in the developing NP [55]. Immunolocalization in 12- to 14-week-old human fetal tissues further confirms that Pln is mainly distributed in NP and internal annulus fibrosus (AF) but shows weak expression in the tissues around the developing IVD and exhibits no expression in the notochordal tissues [64]. Interestingly, Hayes and Melrose recently detected Pln expression in the cytoplasm of both the NP and AF of the sheep IVD and proposed that Pln might be trafficked by transport vesicles connecting the cell nuclear and extracellular environment, indicating a novel transcriptionally regulatory site for the multifunctional roles Pln plays in the IVD [65,66]. In HSPG2−/− mice, cartilage and vertebral bodies show fibrous invasion from the perichondrium resulting in disruption of the growth plate, suggesting that Pln plays a critical role in maintaining the integrity of the matrix structure [52]. Pln in human fetal spine is substituted with HS chains and the 7D4 CS sulfation motif; however, as the tissue matures, this epitope decreases in frequency and becomes virtually undetectable [67,68]. 4C3, 7D4, and 3B3[–] positive progenitor cell populations play important roles not only in IVD development but also in hematopoiesis, skin morphogenesis, and chondrogenesis [20].
Interactions between Pln and various ECM components contribute to matrix stabilization, cell-cell/ECM ligation and IVD cell involvement in the mechanical sensory processes that regulate tissue homeostasis and remodeling [69,70]. For example, elastin, an important ECM component located within translamellar cross bridges and between adjacent annular layers, plays a functional role in developing spinal tissues [71]. A series of related studies have discovered that elastic fiber-associated proteins fibrillin-1 and latent transforming growth factor beta (TGF-β) binding protein-2 (LTBP-2) are found in the annular lamellae in human fetal IVD and are associated with Pln [71]. Some literature shows that the Pln HS chains may contribute to fibrillin-1 assembly and distribution in the IVD [69] and LTBP-2 also appears to be co-localized with Pln in the outer layer of AF [71]. Notably, in the posterior layer of AF, Pln, and LTBP-2 are strongly co-deposited in the PCM of annular fibrochondrocytes [72]. In addition, Pln and COL VI, co-deposited in the PCM of IVD cells, are able to connect cells and their surrounding matrix into a network structure [73]. Furthermore, Pln can be helpful for chondrogenic differentiation of the developing IVD cells through the combination with FGF-18 and FGFR3 [68]. These results demonstrate that Pln is not only a marker of chondrogenesis [49], but also plays a crucial role in regulating early spinal tissue development.
4. Pln mediated biomechanical and biochemical impact on chondrogenesis
Consisting of five domains, each with its own structure and corresponding functions, Pln serves a multitude of complex biomechanical and biochemical roles in cellular maintenance and signaling pathways. Many domains contain homologous features with other biological components (i.e., laminin chain short arm, LDL receptor), as it is this structural adaptability that facilitates Pln’s ability to bind and interact with a myriad of ligands in the PCM and ECM to activate intracellular signaling pathways as well as help in stabilizing the ECM and BM.
4.1. Biomechanical role of Pln in cartilage
Because mechanical signals are integral to cartilage development, pathophysiology, and regeneration [74], various cellular components have been studied to investigate their roles in creating these mechanical signals. As previously reported, the mechanical distribution in articular cartilage is heterogeneous and anisotropic at the microscopic level [75,76]. Stiffness mapping depicted with AFM indicates that the ratio of the modulus between PCM and ECM in the articular cartilage of different species—including human, porcine, and murine—is usually fixed; however, the modulus of ECM is significantly higher than that of PCM in said species [77]. A similar study examining porcine medial meniscus cartilage has demonstrated that the elastic modulus is significantly elevated in the outer region of the Pln-labeled PCM when compared to the inner region [9]. Interestingly, most studies identify PCM of chondrocytes by COL VI labeling [78–80]; over time, Pln labeling has also been recognized as a reliable method to identify PCM [15]. Notably, regions in the PCM labelled by both COL VI and Pln were found to have lower elastic moduli than those labelled by Col IV alone, indicating that local Pln presence in the PCM lowers the elastic moduli [15]. However, in a murine model of the Schwartz-Jampel syndrome disorder, HSPG2 knockdown results in abnormal PCM organization, altering these matrix properties and decreasing chondrocyte stiffness [81].
It has been shown that PCM may act as a regulator in mechanical-biological signaling transduction in chondrocytes, in which Pln plays an essential role [82–84]. HSPG2 knockdown led to defective PCM formation and early onset of osteoarthritis (OA), whereas the concentration of ECM proteins, including many collagens, increased [85]. Physiologically related hydrostatic loading can significantly increase HSPG2 gene expression in cartilage [86], and these changes vary based on the magnitude or duration of the applied loading. For example, in bovine cartilage, Pln expression is not altered after one cycle of physiologic, cyclic hydrostatic loading administered within one week, but does become increased after two and three cycles of loading [87]. Interestingly, Pln expression sensitivity to mechanical loading varies significantly depending on the age group from which the cartilage was derived. Under dynamic mechanical compression, a downregulation of Pln is observed in aged cartilage while no changes are observed in young cartilage [88]. This age-dependency is speculated to be regulated through Smad2/3P signaling and is associated with the mechanisms that lead to the development of OA [88]. Under mechanical loading, Pln is regulated through its interaction with FGF-2, which facilitates FGF-2’s ability to initiate signal transmission quickly and allows Pln to directly participate in the signaling response during loading [84]. It is thought that, while in the resting state, FGF-2 is blocked on the cell surface and cannot bind to FGFR for signal transduction [84], but ECM deformation under mechanical loading allows the FGF-2-bound Pln to be presented to the cell surface receptor—thus activating downstream signaling pathways.
4.2. Biochemical role of Pln in chondrogenesis
Pln function is regulated by the interactions of the Pln core protein and GAG chains with different molecules in cell proliferation, adhesion, and nutrient metabolism [18,26,89,90]. Specifically, during chondrogenesis, specific growth factors and ligands (shown in Table 1) have been reported to be involved in Pln activity.
FGF
The human and mouse FGF gene families are comprised of 22 members [91,92]. Among these, FGF-2 and FGF-18 have been implicated in cartilage development [93].
FGF-2 has been reported to promote chondrocyte proliferation [94] and protect against development of OA by inhibiting a disintegrin and metallopeptidase with thrombospondin type 1 motif 5 (ADAMTS-5) formation [95]. FGF-2 participates in chondrocyte regulation by binding to FGFR1 and FGFR3—both of which play unique roles in the FGF-2 signaling pathways. The FGF-2/FGFR1 signaling pathway promotes deleterious activities such as matrix metalloproteinase (MMP) activation and inhibition of ECM synthesis, while the FGF-2/FGFR3 signaling pathway increases cell proliferation and ECM production [46,93]. Pln regulates these pathways by interacting with FGFs/FGFRs to form a complex that later contributes to signal transduction [96]. Specifically, the FGF-2/Pln complex acts as a mechanotransducer [84]. As a low affinity receptor for FGF-2, Pln from developing growth plate chondrocytes is able to sequester FGF-2 away from the high affinity receptors on chondrocytes [97]. Immunohistochemical results show that Pln and FGF-2 are bound in all regions of cartilage, including weight-bearing and non-weight-bearing areas as well as the superficial zone and the mid zone of human and porcine cartilage [84]. Pln that is localized to the PCM of chondrocytes in developing growth plate cartilage rudiments contains HS chains and CS chains, each of which plays a distinctive role in binding FGF-2. While FGF-2 is able to freely bind to the HS chain of Pln, it is only able to be delivered to the FGFRs (FGFR1 and FGFR3) when the CS chain of Pln is removed [98].
As a regulator of chondrocyte proliferation, hypertrophy, and vascularization in the growth plate, FGF-18 plays different regulatory roles by binding to various FGFRs [99]. During long bone development, FGF-18 affects chondrogenesis through FGFR3 based signaling and osteogenesis through FGFR1 and/or FGFR2 based signaling [100,101]. In a cell culture system containing fetal rudiment chondrocytes, exogenous FGF-18 is able to enhance Pln expression in cartilaginous matrices [102]. PlnDIII is involved in binding FGF-18, and by doing so, Pln can change the mitogenic effect FGF-18 has on chondrocytes in the growth plate [103]. It has also been found that the HS chains of Pln modulate complex formation between FGF-18, FGFR3, and Pln; without FGF-18 participation, FGFR3 alone is unable to directly bind to the HS chains of Pln [48].
Other FGFs, such as FGF-9, are also capable of contributing to chondrogenesis. One instance can be seen with the sequential addition of FGF-2, FGF-9, and FGF-18, which helps to enhance the cartilage differentiation capabilities of human mesenchymal stem cells (MSCs) [104]. Interestingly, chondrocyte Pln bound FGF-1 and -9 appear to be less versatile than endothelial cell Pln. When examining BaF-32 cells transfected with FGFR3c, endothelial cell-derived Pln supports cell proliferation equally well with FGF-1 and -9, while chondrocyte-derived Pln only supports BaF-32 cell proliferation through the FGF-9/FGFR3c complex [49].
BMP-2
BMP-2 can effectively enhance MSC recruitment to cartilage condensation and initiate a BMP-dependent signaling pathway to induce chondrogenesis of mesenchymal progenitor cells [105]. As such, exposure to BMP-2 has been well documented to be important for articular cartilage development, maintenance, improved MSC chondrogenesis, and normal joint formation [106,107].
The interactions between BMP-2 and Pln have been shown to contribute to cartilage development. For example, C3H10T1/2 cells enter chondrogenic differentiation when plated on intact Pln or independent PlnDI; however, these Pln-stimulated cellular nodules fail to express chondrogenic maturation and subsequent terminal differentiation markers unless they undergo treatment with BMP-2 [40,108]. Thus, it is possible that BMP-2 treatment enhances the extracellular-signal-regulated kinase 1/2 (ERK1/2) activity involved in chondrogenesis of C3H102T1/2 cells [109]; alternatively, HS chains of Pln can improve the biological activity of BMP-2 [110] and be more conducive to chondrogenesis than adipogenesis [108]. BMP-2 binding to PlnDI HS chains is necessary for coordinated chondrogenic differentiation [111]. In addition, the close combination of BMP-2 and PlnDI also improves osteogenic ability [112]. These functions are thought to stem from PlnDI’s ability to act as a repository for BMP-2 storage, thus allowing for its controlled release, protecting it from degradation, and enhancing its biological activity [113,114].
CTGF
As a multifunctional secretory ECM protein with four domains, CTGF is highly expressed on hypertrophic chondrocytes in growing cartilage [115]. A myriad of studies have suggested that CTGF may play an integral role in many biological processes, affecting proliferation, angiogenesis, migration, differentiation, cell adhesion, wound healing, and cell-specific ECM protein synthesis [116–119]. Notably, despite being a hypertrophic chondrocyte gene product, CTGF itself does not stimulate articular chondrocyte hypertrophy [120]. In 2003, Nishida et al. found that CTGF co-localizes with Pln from chondrosarcoma derived HCS2/8 cells and that recombinant CTGF is able to dramatically promote HSPG2 gene expression in vitro [28]. As CTGF is produced by hypertrophic chondrocytes in the hypertrophic region and Pln is also predominantly localized to the pre-hypertrophic region, it is possible that the role that CTGF plays in chondrocyte proliferation and differentiation may occur through Pln [28]. Outside of its interactions with Pln, CTGF is also capable of regulating chondrocyte proliferation and differentiation via BMP and mitogen-activated protein kinase (MAPK) signaling pathways [121].
WARP
Encoded by the VWA1 gene, WARP is a member of the von Willebrand factor A domain protein family in the ECM [122]. Though previous research claims that WARP is primarily expressed in articular cartilage and the IVD [31], immunohistochemical studies have shown that the distribution of WARP varies with cartilage development. During mouse embryonic development, WARP distribution in articular cartilage is restricted to the chondrogenous layers at embryonic day E15.5 and the PCM in the sixth week [31]. In the spine, WARP distribution is restricted to the articular surfaces of the vertebrae and PCM in the AF at E18.5 [31]. Besides co-localization with Pln by binding to domain III-2 core protein and domain I HS chains in cartilage [31], WARP also co-localizes with COL VI in the PCM of the superficial zone of articular chondrocytes [123]. This finding suggests that WARP may act as a bridge of communication between Pln and COL VI-labeled PCM in regulating human articular cartilage [123].
Outside of its role in cartilage, WARP is also expressed and co-stained with Pln in some non-cartilaginous tissues, including the vasculature of the central nervous system and muscle tissues, suggesting that WARP may help to maintain the function of a wide variety of Pln-containing tissues [124]. However, a study published in 2009 found that the absence of WARP does not cause abnormalities or affect BM formation in articular cartilage, IVD, or muscle tissue, rather it affects only the structure and function of the peripheral nervous system [125]. These studies indicate that WARP plays a specialized role in some BM structures, such as vasculature, muscle, and cartilage [126].
4.3. Roles of the Pln CS chain in chondrogenesis
Despite the fact that Pln is usually substituted with HS chains in vascular tissues [127], sometimes it is substituted with a hybrid form of HS and CS chains in chondrocytes and smooth muscle cells [128]. Infrequently, epithelial cell and chondrocyte-derived Pln is also a hybrid Pln and a unique PG containing HS, CS, and keratan sulfate (KS) chains [48,129]. CS sulfation motifs play important roles in the development of the human fetal hip, knee, and elbow joints and related connective tissues [20,130,131]. The fetal IVD progenitor cells also contain Pln changeably substituted with native 7D4 CS sulfation motif [67]. By decorating cell surface PGs on activated stem/progenitor cells, the CS sulfation motifs 4C3, 7D4, and 3B3[–] can be used to identify these cells in transitional areas of tissue development and in repair tissues [20,132]. Through binding, sequestration, or presentation of bioactive signaling molecules, such as FGF, CS sulfation motifs participate in modulating the signaling gradients in charge of the cellular behaviors, including but not limited to differentiation, proliferation, and matrix turnover, which shape the zonal tissue architecture existing in mature articular cartilage [130].
5. Signaling pathways of Pln and effect on cartilage development
Pln’s unique localization and binding interactions induce many intricate signaling pathways with downstream targets that significantly impact chondrocyte gene expression and the surrounding mechanotransduction. (Figure 2) As such, Pln is seen to play an integral role in regulating cartilage behavior throughout its genesis and development, including but not limited to: prechondrogenic cell behavior, catabolic and anabolic regulation of cartilage homeostasis, chondrocyte proliferation and differentiation, and cartilage tissue vascularization.
Figure 2.
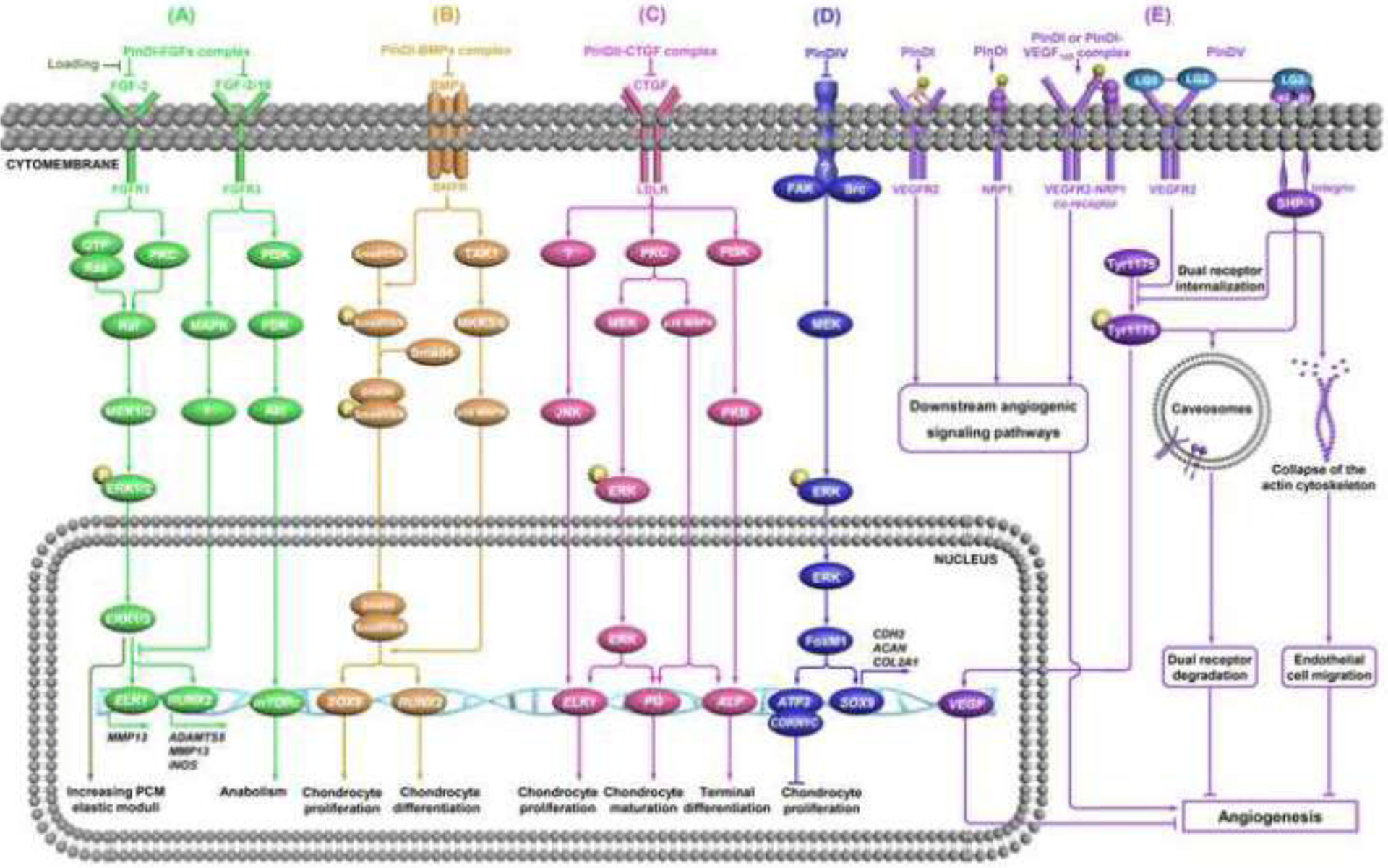
Signaling pathways of Pln and effect on cartilage development. (A) FGF signaling and mechanotransduction role. The role Pln plays in regulating cartilage homeostasis is achieved by the formation of the PlnDI-FGF complex. This complex sequesters FGFs from receptors on the cell surface and inhibits the downstream signaling pathway [48,93,135–137]. FGF-2 also plays a mechanotransduction role in cartilage via influencing the ERK signaling pathway [15,84,134,139]. (B) BMP signaling. The roles that BMP signaling plays in chondrogenesis and osteogenesis are regulated by BMP-2 storage in the PlnDI-BMP complex and the delicate balance between ligands and antagonists [143–147,149]. (C) CTGF signaling. The interaction between CTGF and PlnDII triggers various intracellular signaling pathways and thus regulates the proliferation and maturation of chondrocytes [29,121,151,152,154,155]. (D) FAK-Src signaling. PlnDIV prevents early cell attachment and promotes cell clustering/condensation by suppressing the FAK/Src signaling pathway, but the receptor is unknown [37,156,157]. (E) VEGF signaling. In the early stage of cartilage development, PlnDI, unbound or bound to VEGF165, stimulates angiogenesis by direct interactions with neuropilin 1 (NRP1), VEGFR2, and NRP1/VEGFR2 co-receptors [164]. In the later stage of cartilage development, PlnDV inhibits angiogenesis via dual receptor VEGF2 and integrin. The endorepellin-induced dual receptor internalization and degradation by the caveosome-mediated pathway is also involved in angiostasis [163,165,171].
5.1. FGF signaling and mechanotransduction role
Current studies have shown that Pln plays a key role in post-traumatic OA progression. The ablation of PlnDI HS could inhibit articular cartilage degradation, decrease synovial inflammation, and reduce osteophyte size [46]. In cartilage, Pln not only participates in FGF delivery to cell surface receptors, but also sequesters FGFs from the PCM, acting as a mechanotransducer during cartilage loading [15,84] or in response to cartilage injury [133,134].
Two members of the FGF family, FGF-2 and FGF-18, are important regulators of cartilage homeostasis. It has been demonstrated that chondrocyte-derived Pln HS regulates cartilage homeostasis by participating in tripartite complex formation with both FGF-18 and FGFR3 as well as FGF-2 and FGFR1 or FGFR3 [48]. FGF-2 selectively activates FGFR1 to upregulate the production of matrix-degrading enzymes and inhibit the synthesis of ECM and PGs [135,136]. Thus, in adult human articular chondrocytes, FGF-2 takes on a catabolic role [93]. The interactions between FGF-2 and FGFR1 independently activate Ras and protein kinase C (PKC), both of which are integrated by Raf-MEK1/2-ERK1/2 and thus concertedly drive signaling [93,137]. ERK1/2 activates at least two key downstream transcription factors [i.e., Ets-like protein-1 (ELK1) and runt-related transcription factor 2 (RUNX2)] that upregulate the expression of a series of matrix-degrading enzymes [137]. On the other hand, FGF-18 binds to FGFR3 and then activates downstream MAPK and protein kinase B (Akt) signal pathways [93,138]. The MAPK signal pathway can inhibit ELK1 and RUNX2 expression which decreases ECM degradation, while the Akt pathway promotes ECM formation and chondrocyte differentiation [93]. Hence, the downstream effects of FGF-18 ultimately promote anabolic activity in human articular chondrocytes [93].
FGF-2 has been shown to influence Pln-regulated mechanotransduction in cartilage via the ERK signaling pathway [84,134,139]. The elastic moduli of the PCM is lower in Pln-rich regions than in Pln-absent regions. This lower elastic modulus is thought to arise from the Pln HS chain interactions, as the enzymatic removal of HS chains drastically enhances PCM’s microscale elastic properties while exhibiting little influence on ECM properties [15]. In unloaded cartilage, the Pln HS chain bound FGF-2 is sequestered from the cell surface, but upon matrix deformation, HS-bound FGF-2 is presented to its corresponding cell surface receptor, thus activating the ERK signaling pathway [84]. Besides this mechanism, other pathways have also been documented to contribute to the chondrocyte response to loading. For instance, in 2007, loading-induced protein tyrosine phosphorylation was found to be FGF dependent [84]; briefly, the authors found that FGF-2 and perlecan co-located in the PCM of articular cartilage; ERK activation upon loading was dependent on pericellular FGF-2 rather than release of intracellular growth factors. They also found that FGFR inhibitor could markedly suppress the increase in the level of protein tyrosine phosphorylation upon loading. These findings indicate that FGF-2/Pln is involved in signal transduction under stress stimulation.
5.2. BMP signaling
BMP-2 is essential to bone and cartilage formation [140]. The interactions between BMP-2 and Pln have been shown to contribute to cartilage development [111,113,141] and improve osteogenic ability [112]. BMP-mediated developmental processes are known to depend on the basic N-terminal domains of dimeric BMP-2 that serve as heparin-binding sites; although these sites are not integral to receptor co-activation, they help to regulate its biological activity [142]. Jiao et al. demonstrated that HSPG modulates the osteogenic activity of BMP-2 by sequestering BMP-2 at the cell surface so that it is unable to bind to the BMP receptor [143]. PlnDI can thus serve as a depot for BMP-2 storage, allowing it to be released in a controlled manner, protecting it from degradation, and enhancing its biological activity [113,114].
Both the canonical Smad-dependent pathway—involving TGF-β/BMP ligands, receptors, and Smads—and the non-canonical Smad-independent pathway—involving p38 MAPK—have been identified in chondrocytes [144,145]. Most BMPs phosphorylate and activate R-Smads (Smad1/5/8) to complex with Co-Smad (Smad4); the Smad1/5/8-Smad4 complex subsequently translocates into the nucleus to regulate RUNX2 and SRY (sex determining region Y)-box 9 (SOX9) target gene expression [146,147]. Interestingly, p38 MAPK is also involved in fine-tuning BMP’s impact on chondrocytes and osteoblasts through TGF-β-activated kinase 1 (TAK1) [148]. In the postnatal chondrocyte, TAK1 deletion exhibits severe growth retardation and reduced SOX protein, PG, and COL II expression [149]. In contrast, in osteoblasts, TAK1 deletion leads to clavicular hypoplasia and postpones fontanelle fusion, resulting in a phenotype alike to that seen in cleidocranial dysplasia—a disorder resulting from RUNX2 deficiency [150].
The roles that BMP signaling plays in chondrogenesis and osteogenesis are regulated by the delicate balance between ligands and antagonists—including Chordin, Chordin-like (CHL), Fellistatin, gremlin 1 (Grem1), and Noggin [144]—and these BMP antagonist expression patterns vary depending on joint location and species [106].
5.3. CTGF signaling
As a multifunctional secretory ECM protein, CTGF is highly expressed by hypertrophic chondrocytes in growth cartilage [115]. Not only does CTGF co-localize with Pln from HCS2/8 cells, but recombinant CTGF can also promote the in vitro expression of the HSPG2 gene dramatically [29]. The LDL receptor is known to directly interact with CTGF [151,152]. Recently, it has been shown that PlnDII, which contains repeat sequences highly similar to those of the LDL receptor, can also bind CTGF in the PCM of articular cartilage [153]. As such, CTGF may be able to promote chondrocyte proliferation and differentiation through its interaction with Pln [29].
The interaction between CTGF and Pln triggers various intracellular signaling pathways. PKC acts as a central upstream kinase in chondrocyte proliferation, maturation, and terminal differentiation through its triggering of MEK, p38 MAPK, and PKB, respectively [121]. CTGF also activates the JNK pathway, which triggers chondrocyte proliferation and maturation [121]. In promoting chondrocyte terminal differentiation, PKB-mediated activation of phosphoinositide 3-kinase (PI3K) is also required [121,154,155].
5.4. FAK-Src signaling
PlnDIV prevents early cell attachment and promotes cell clustering by suppressing focal adhesion kinase/steroid receptor co-activator (FAK/Src) activity, with increased mRNA levels of chondrogenic markers SOX9, Cadherin 2 (CDH2), type II collagen (COL2A1), and aggrecan (ACAN) [37]. These markers are uniquely indicative of different milestones in chondrogenic differentiation. For instance, CDH2 is a precartilage condensation marker and downstream target of SOX9 [156]. The regulatory role that FAK-Src signaling plays in cell adhesion and spreading occurs via the loss of phospho-ERK1/2 [37]. Forkhead box protein M1 (FoxM1), a downstream target of ERK, is involved in cell cycle progression and is downregulated as a result of PlnDIV-induced clustering, along with increased expression of cyclin-dependent kinase inhibitor 1C (CDKN1C) and activating transcription factor 3 (ATF3) [37]. Overexpression of ATF3 in chondrocytes then results in downregulation of cyclin D1 and cyclin A expression [157]. As such, the FAK-Src pathway culminates with chondrocyte proliferation suppression [37]. These findings described the mechanism of how PlnDIV affects prechondrogenic cell condensation and prevents chondrocyte progression to fibrocartilage.
5.5. VEGF signaling
In the early stages of cartilage development, angiogenesis helps to provide nutrients to the immature cartilage. As a component of vascular BM, Pln contributes to the process of angiogenesis [158] and wound healing [159]. Furthermore, studies have pointed out that VEGF is necessary in cartilage angiogenesis during cartilage development and chondrocyte differentiation during endochondral bone formation [160–162]. Thus, regulation of VEGF expression is important in maintaining the chondrocyte phenotype. Pln can play promoting and/or inhibiting roles during angiogenesis [163–165], and VEGFR1 and VEGFR2 are the primary receptors involved in angiogenic activities of VEGF [166]. Early studies have shown that full-length Pln and immobilized forms of PlnDI can adjust the VEGF-VEGFR2 signaling pathway to regulate developmental angiogenesis [167,168]. Muthusamy and colleagues later found that the soluble forms of recombinant PlnDI, unbound or bound to VEGF165, stimulate angiogenesis by direct interactions with: 1) neuropilin 1, 2) VEGFR2, and 3) neuropilin 1 and VEGFR2 co-receptors [164]. It was later proven that cartilage Pln is a necessary component in the activation of VEGFR signaling in endothelial cells that ultimately leads to vascular invasion into the cartilage [169]. As Pln is a component of the surface vessel network, which provides nutrients to the developing joint rudiment, Pln regulation is likely beneficial to the activation of angiogenesis in early cartilage development [47].
The C-terminal fragment of Pln (PlnDV), called Endorepellin, contains three laminin globular domains (LG1/2/3) and is responsible for facilitating the role Pln plays in inhibiting angiogenesis [163,165,170]. LG1/2 domains bind to VEGFR2, inhibiting its VEGF-mediated activation by blocking Tyr1175 phosphorylation. This inhibition blocks endothelial cell migration and VEGF gene transcription, both of which contribute to angiostasis [165]. The LG3 domain, on the other hand, directly binds to α2β1 integrin, which also contributes to anti-angiogenesis [171]. The mechanism involves the activation of Src homology-2 protein phosphatase-1 (SHP-1), which dephosphorylates Tyr1175 of VEGFR2 and induces cytoskeletal collapse [171]. Both VEGFR2 and α2β1 integrin are required for Endorepellin angiostatic activity and the internalization and degradation of the dual receptor is mediated by the caveosome pathway [163,165].
6. Potential applications and challenge
Pln has been shown to be not only responsible for much of the biomechanics that characterize its matrices, as well as displaying cell source-dependent functionality that affects its ability to facilitate matrix mechanics. This structural variability lends Pln great potential for use in biomaterial synthesis. To this end, we explore current literature to speculate about the potential involvement of Pln in cartilage regeneration following injury and potential applications of Pln in stem cell and biomaterial mediated cartilage engineering.
6.1. Pln may mediate cartilage regeneration following injury
Given its impact on angiogenesis, wound healing, and maintaining cartilage function, Pln might be positively associated with cartilage regeneration. A study analyzing fresh bovine cartilage specimens found a significant negative correlation between age and HSPG2 mRNA expression [87]. Interestingly, in human knee OA cartilage, Pln levels are significantly higher in areas close to cartilage defects [172]. Similarly, stimulation of equine cartilage with IL-1β has been proven to upregulate Pln expression [173]. Furthermore, a 2015 study found that HSPG breakdown during inflammatory and mechanical injury in serious cases of OA leads to the disruption of the ELR+ CXC chemokine signaling pathway, contributing to loss of chondrocyte phenotypic stability. This process is due to HSPG’s function in retaining C-X-C motif chemokine ligand 6 (CXCL6), a compound that has the capacity to attract inflammatory cells when released from the ECM and is linked to inflammatory arthritis [174]. Pln’s chondroprotective role is thought to stem from Pln’s ability to modulate FGF signaling [84,175].
Much evidence also points to the potential utilization of Pln as a regulator in stem cell chondrogenic differentiation. As mentioned above, GAG chains of Pln can interact with various ligands to regulate chondrogenesis. Exogenous HS has been proven to enhance TGF-β3-induced chondrogenic differentiation of human MSCs by triggering the Smad2/3 signaling pathway [176]. Furthermore, a series of studies conducted by Sadatsuki and colleagues concluded that Pln in the synovial niche is essential for chondrogenesis of synovial mesenchymal cells, but not for osteogenesis and cell proliferation [177]. This Pln-induced synovial mesenchymal cell chondrogenic differentiation primarily depends on the regulation of SOX9 gene expression [178] as well as contributions from the Smad and MAPK signaling pathways [179].
Despite the promotion of developing and mature cartilage tissues, Pln also retains permanent cartilage by preventing further development, indicative of its role in tissue homeostasis. Shu and coworkers found that the use of heparanase in chondrocyte cultures increased cell proliferation and GAG production. Furthermore, they found that growth plate maturation, GAG deposition, and cell proliferation and maturation significantly enhanced in the Pln exon 3 null mouse model IVD, demonstrating the repressive control of the Pln HS chain [10]. The function of the Pln HS chain in tissue stabilization was also found in the PCM of AF cells and chondrocytes through interaction with COL XI [180] and in the small blood vessels of the synovium via interaction with elastin and fibrinlin-1 [181].
6.2. Pln potential applications in scaffold design
Ideal characteristics of a biomaterial that is to be used in cartilage regeneration necessitate the material’s biocompatibility, bioactivity, biomimetics, biodegradability, and bioresponsiveness [182]. Given Pln’s unique structure, localization, and distinctive biological activity displayed by each of the five different domains, some scholars [183] believe that it is an ideal candidate for modifying and enhancing the extracellular scaffold, directly linking the ECM to the cell surface and initiating cellular signals. Domain I, in particular, is recognized to have well-defined functionality in cartilage regeneration. Previous research shows that chitosan scaffolds containing PlnDI have the potential to induce wound healing [159]. PlnDI-COL II fibril modified polylactic acid scaffolds support chondrogenesis of both primary mouse embryonic fibroblasts and C3H10T1/2 cells via the binding and retention and release of BMP-2 [141]. Electrospun collagen fibers coated with PlnDI are able to bind to FGF-2 at rates that are ten times that of heparin-bovine serum albumin collagen fiber; this finding demonstrates a potential application of Pln-enhanced materials in constructing tissue engineering scaffolds [184]. Furthermore, combining a hyaluronic acid-based hydrogel with PlnDI has proven to make up an effective system for BMP-2 delivery that is able to promote chondrogenic differentiation in vitro [111] and serve as an injectable therapeutic agent for OA treatment in vivo [113]. As indicated in Table 2, growing evidence suggests that Pln functionality is not limited to use in cartilaginous tissues, but rather it has broad application in enhancing biomaterials for cell-based therapies.
Table 2.
Positive effects of perlecan on biomaterials improvement.
| Species | Cell/tissue location | Modified biomaterials | Result | References |
|---|---|---|---|---|
| Human | MG-63 cell | Coating electrospun collagen and gelatin fibers with PlnDI | Increasing FGF-2 binding | [184] |
| BMSC/MG-63 cell | PlnDI-containing COL I scaffold | Promoting cell proliferation by enhancing FGF-2 activity | [204] | |
| C4-2B cell | Electrospun PCL-based scaffolds with PlnDIV | Facilitating cell proliferation, survival, and migration | [205] | |
| Coronary artery endothelial cell | Silk biomaterials functionalized with PlnDV | Supporting cell adhesion, spreading, and proliferation | [206] | |
| Mouse | Cartilage | PlnDI immobilized to HA microgel | Repairing OA-like damage by controlled release of BMP-2 | [113] |
| C3H10T1/2 cell | PlnDI-conjugated, HA-based HGPs | Stimulating chondrogenic specific ECM production by binding and sustained release of BMP-2 | [111] | |
| PlnDI and COL II coated PLA scaffold | Promoting cartilage-like tissue in PlnDI/COL II coated PLA scaffold | [141] | ||
| BMSC | PlnDI-functionalized electrospun PCL scaffolds | Enhancing ALP activity by increasing PCL loading capacity of BMP-2 | [207] | |
| RGC-5 cell | Biological glue made by mixing laminin, COL IV, entactin, and Pln | Improving retina adhesion rate | [208] | |
| Rat | Skin | PlnDI-containing chitosan scaffolds | Promoting angiogenesis and dermal wound healing | [159] |
| Bone | A recombinant PlnDI expressed from HEK 293 cells with HS/CS PG tightly binding recombinant human BMP-2 | Promoting dose-enhancement of BMP-2 on a particulate TCP scaffold to generate up to 9 fold more bone volume in 6 or more weeks in a rat model than the control without PlnDI | [112] |
Abbreviation: ALP: alkaline phosphatase; BMP-2: bone morphogenetic protein 2; BMSC: bone marrow stromal cell; C3H10T1/2: a murine mesenchymal stem cell line; C4-2B: a cancer cell line; CS: chondroitin sulfate; ECM: extracellular matrix; FGF-2: fibroblast growth factor 2; HA: hyaluronic acid; HGP: hydrogel particle; HS: heparan sulfate; MG63: an osteoblastic cell line; OA: osteoarthritis; PCL: poly (epsilon-caprolactone); PLA: polylactic acid; PG: proteoglycan; RGC-5: a retinal ganglion cell line; TCP: tricalcium phosphate
7. Conclusions and perspective
As a key component of stem cell niches, Pln is part of a highly complex microenvironment that promotes cartilage regeneration [131], which is supported by the presence of Pln in repaired human cartilage tissue through cell therapy and natural occurrence in a recent report [185]. In this review, we have compiled growing evidence that Pln in the PCM of cartilaginous tissues is involved in the regulation of biomechanical properties that characterize its various matrices and may thus have unique application in cartilaginous tissue regeneration. Although increasing evidence suggests that the PCM may serve as a mechanical-biological signal transducer in chondrocytes, the specific role that Pln plays in various mechanical-biological signaling pathways is still unclear. Based on the features mentioned above, Pln appears to have great potential for improving the cell culture environment. However, current efforts to introduce Pln to tissue engineering have been limited to the use of Pln-coating in cell culture environments—such applications prove to be insufficient given Pln’s potential as a biomaterial. Given that cell-derived decellularized ECM—an excellent in vitro 3D culture scaffold model—can rejuvenate stem cell proliferation and chondrogenic differentiation [186–188], which has been used to define the impact of fibronectin on stem cells’ proliferation and differentiation [189], it might provide an excellent in vitro model to delineate the role Pln plays in this matrix microenvironment.
Despite various efforts to illuminate Pln’s role in chondrogenesis and cartilage regeneration, our understanding of Pln function remains limited. PlnDI is unique to Pln and many studies focus on this domain; however, the functions of other domains of Pln lack attention, which might be the focus of future investigation. In practical terms, the reproduction of specific HS sequence information on recombinant Pln domains is technically problematic especially since the specific HS sequences that drive repair are incompletely understood, despite that recombinant PlnDI and PlnDV forms containing GAG have been prepared/used successfully in a number of tissue repair strategies [111,113,184,190,191].
Besides cartilage regeneration, the role of Pln in cartilage degeneration also deserves attention. For instance, a 2013 study using a synovial HSPG2-deficient mouse model found synovium-localized Pln to be required in osteophyte formation in OA [192]. Furthermore, a 2015 study found that blocked endogenous Pln function improves differentiation of rat articular chondrocytes in vitro, suggesting that Pln may promote the dedifferentiation of chondrocytes; it should be noted that this result is only seen in environments favoring dedifferentiation (i.e., canonical Wnt signaling and low levels of BMP-2 and BMP-4) [193]. Because the degenerative contribution of Pln has only been observed in experiments testing a pre-existing degenerative microenvironment, it is likely that Pln only serves a passive role in cartilage degeneration. Hence, evidence that Pln actively triggers catabolic responses is still lacking and deserves further study.
Acknowledgements
We thank Suzanne Danley for editing the manuscript. This work was supported by Research Grants from the National Institutes of Health (1R01AR067747) to M.P. and Health Commission of Sichuan Province (18PJ008) and Science & Technology Department of Sichuan Province (2019YFS0267) to S.C.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could appear to influence the work reported in this paper.
References
- 1.Chen S, Fu P, Wu H, Pei M, Meniscus, articular cartilage and nucleus pulposus: a comparative review of cartilage-like tissues in anatomy, development and function, Cell Tissue Res. 370 (2017) 53–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hunziker EB, Articular cartilage structure in humans and experimental animals. In: Kuettner KE, Schleyerback R, Peyron JG, Hascall VC, Eds. Articular Cartilage and Osteoarthritis. New York: Raven Press; (1992) 183–199. [Google Scholar]
- 3.Timpl R, Brown JC, Supramolecular assembly of basement membranes, BioEssays 18 (1996) 123–132. [DOI] [PubMed] [Google Scholar]
- 4.LeBleu VS, Macdonald B, Kalluri R, Structure and function of basement membranes, Exp. Biol. Med 232 (2007) 1121–1129. [DOI] [PubMed] [Google Scholar]
- 5.Erickson AC, Couchman JR, Still more complexity in mammalian basement membranes, J. Histochem. Cytochem 48 (2000) 1291–1306. [DOI] [PubMed] [Google Scholar]
- 6.Yurchenco PD, Cheng YS, Campbell K, Li S, Loss of basement membrane, receptor and cytoskeletal lattices in a laminin-deficient muscular dystrophy, J. Cell Sci 117 (2004) 735–742. [DOI] [PubMed] [Google Scholar]
- 7.Kvist AJ, Nystrom A, Hultenby K, Sasaki T, Talts JF, Aspberg A, The major basement membrane components localize to the chondrocyte pericellular matrix--a cartilage basement membrane equivalent? Matrix Biol. 27 (2008) 22–33. [DOI] [PubMed] [Google Scholar]
- 8.Njoto I, Fatchiyah F, Handono K, Abdurrachman A, Soeatmadji DW, Kalim H, Modulation of Perlecan Protein towards Chondrocyte Secretion Factors at the Articular Cartilage in Hyperglycemic Animal Model, J. Pure App. Chen. Res 8 (2019) 80–86. [Google Scholar]
- 9.Sanchez-Adams J, Wilusz RE, Guilak F, Atomic force microscopy reveals regional variations in the micromechanical properties of the pericellular and extracellular matrices of the meniscus, J. Orthop. Res 31 (2013) 1218–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shu CC, Smith SM, Little CB, Melrose J, Elevated hypertrophy, growth plate maturation, glycosaminoglycan deposition and exostosis formation in the Hspg2 exon 3 null mouse intervertebral disc, Biochem. J 476 (2019) 225–243. [DOI] [PubMed] [Google Scholar]
- 11.Arikawa-Hirasawa E, Wilcox WR, Le AH, Silverman N, Govindraj P, Hassell JR, Yamada Y, Dyssegmental dysplasia, Silverman-Handmaker type, is caused by functional null mutations of the perlecan gene, Nat. Genet 27 (2001) 431–434. [DOI] [PubMed] [Google Scholar]
- 12.Trout AL, Rutkai I, Biose IJ, Bix GJ, Review of Alterations in Perlecan-Associated Vascular Risk Factors in Dementia, Int. J. Mol. Sci 21 (2020) 679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arikawa-Hirasawa E, Le AH, Nishino I, Nonaka I, Ho NC, Francomano CA, Govindraj P, Hassell JR, Devaney JM, Spranger J, et al. Structural and functional mutations of the perlecan gene cause Schwartz-Jampel syndrome, with myotonic myopathy and chondrodysplasia, Am. J. Hum. Genet 70 (2002) 1368–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pei S, Parthasarathy S, Parajuli A, Martinez J, Lv M, Jiang S, Wu D, Wei S, Lu XL, Farach-Carson MC, et al. Perlecan/Hspg2 deficiency impairs bone’s calcium signaling and associated transcriptome in response to mechanical loading, Bone 131 (2020) 115078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilusz RE, Defrate LE, Guilak F, A biomechanical role for perlecan in the pericellular matrix of articular cartilage, Matrix Biol. 31 (2012) 320–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gomes R, Kirn-Safran C, Farach-Carson MC, Carson DD, Perlecan: an important component of the cartilage pericellular matrix, J. Musculoskelet. Neuronal interact 2 (2002) 511–516. [PMC free article] [PubMed] [Google Scholar]
- 17.Gomes RR Jr, Farach-Carson MC, Carson DD, Perlecan functions in chondrogenesis: insights from in vitro and in vivo models, Cells Tissues Organs 176 (2004) 79–86. [DOI] [PubMed] [Google Scholar]
- 18.Knox SM, Whitelock JM, Perlecan: how does one molecule do so many things? Cell. Mol. Life Sci 63 (2006) 2435–2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martinez JR, Dhawan A, Farach-Carson MC, Modular Proteoglycan Perlecan/HSPG2: Mutations, Phenotypes, and Functions, Genes (Basel) 9 (2018) 556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hayes AJ, Hughes CE, Smith SM, Caterson B, Little CB, Melrose J, The CS sulfation motifs 4C3, 7D4, 3B3[-]; and perlecan identify stem cell populations and their niches, activated progenitor cells and transitional areas of tissue development in the fetal human elbow, Stem Cells Dev. 25 (2016) 836–847. [DOI] [PubMed] [Google Scholar]
- 21.Kvist AJ, Johnson AE, Mörgelin M, Gustafsson E, Bengtsson E, Lindblom K, Aszódi A, Fässler R, Sasaki T, Timpl R, Aspberg A, Chondroitin sulfate perlecan enhances collagen fibril formation. Implications for perlecan chondrodysplasias, J. Biol. Chem 281 (2006) 33127–33139. [DOI] [PubMed] [Google Scholar]
- 22.Hassell JR, Robey PG, Barrach HJ, Wilczek J, Rennard SI, Martin GR, Isolation of a heparan sulfate-containing proteoglycan from basement membrane, Proc. Nat. Acad. Sci. U.S.A 77 (1980) 4494–4498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kallunki P, Tryggvason K, Human basement membrane heparan sulfate proteoglycan core protein: a 467-kD protein containing multiple domains resembling elements of the low density lipoprotein receptor, laminin, neural cell adhesion molecules, and epidermal growth factor, J. Cell Biol 116 (1992) 559–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murdoch AD, Dodge GR, Cohen I, Tuan RS, Iozzo RV, Primary structure of the human heparan sulfate proteoglycan from basement membrane (HSPG2/perlecan). A chimeric molecule with multiple domains homologous to the low density lipoprotein receptor, laminin, neural cell adhesion molecules, and epidermal growth factor, J. Biol. Chem 267 (1992) 8544–8557. [PubMed] [Google Scholar]
- 25.Noonan DM, Fulle A, Valente P, Cai S, Horigan E, Sasaki M, Yamada Y, Hassell JR, The complete sequence of perlecan, a basement membrane heparan sulfate proteoglycan, reveals extensive similarity with laminin A chain, low density lipoprotein-receptor, and the neural cell adhesion molecule, J. Biol. Chem 266 (1991) 22939–22947. [PubMed] [Google Scholar]
- 26.Iozzo RV, Basement membrane proteoglycans: from cellar to ceiling, Nat. Rev. Mol. Cell Biol 6 (2005) 646–656. [DOI] [PubMed] [Google Scholar]
- 27.Melrose J, Perlecan, a modular instructive proteoglycan with diverse functional properties, Int. J. Biochem. Cell Biol 128 (2020) 105849. [DOI] [PubMed] [Google Scholar]
- 28.Hummel S, Osanger A, Bajari TM, Balasubramani M, Halfter W, Nimpf J, Schneider WJ, Extracellular matrices of the avian ovarian follicle. Molecular characterization of chicken perlecan, J. Biol. Chem 279 (2004) 23486–23494. [DOI] [PubMed] [Google Scholar]
- 29.Nishida T, Kubota S, Fukunaga T, Kondo S, Yosimichi G, Nakanishi T, Takano-Yamamoto T, Takigawa M, CTGF/Hcs24, hypertrophic chondrocyte-specific gene product, interacts with perlecan in regulating the proliferation and differentiation of chondrocytes, J. Cell Physiol 196 (2003) 265–275. [DOI] [PubMed] [Google Scholar]
- 30.Tiedemann K, Sasaki T, Gustafsson E, Göhring W, Bätge B, Notbohm H, Timpl R, Wedel T, Schlötzer-Schrehardt U, Reinhardt DP, Microfibrils at basement membrane zones interact with perlecan via fibrillin-1, J. Biol. Chem 280 (2005) 11404–11412. [DOI] [PubMed] [Google Scholar]
- 31.Allen JM, Bateman JF, Hansen U, Wilson R, Bruckner P, Owens RT, Sasaki T, Timpl R, Fitzgerald J, WARP is a novel multimeric component of the chondrocyte pericellular matrix that interacts with perlecan, J. Biol. Chem 281 (2006) 7341–7349. [DOI] [PubMed] [Google Scholar]
- 32.Mongiat M, Taylor K, Otto J, Aho S, Uitto J, Whitelock JM, Iozzo RV, The protein core of the proteoglycan perlecan binds specifically to fibroblast growth factor-7, J. Biol. Chem 275 (2000) 7095–7100. [DOI] [PubMed] [Google Scholar]
- 33.Smith SM, West LA, Hassell JR, The core protein of growth plate perlecan binds FGF-18 and alters its mitogenic effect on chondrocytes, Arch. Biochem. Biophys 468 (2007) 244–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cunningham BA, Hemperly JJ, Murray BA, Prediger EA, Brackenbury R, Edelman GM, Neural cell adhesion molecule: structure, immunoglobulin-like domains, cell surface modulation, and alternative RNA splicing, Science 236 (1987) 799–806. [DOI] [PubMed] [Google Scholar]
- 35.Noonan DM, Hassell JR, Perlecan, the large low-density proteoglycan of basement membranes: structure and variant forms, Kidney Int. 43 (1993) 53–60. [DOI] [PubMed] [Google Scholar]
- 36.Ledbetter SR, Fisher LW, Hassell JR, Domain structure of the basement membrane heparan sulfate proteoglycan, Biochemistry 26 (1987) 988–995. [DOI] [PubMed] [Google Scholar]
- 37.Martinez JR, Grindel BJ, Hubka KM, Dodge GR, Farach-Carson MC, Perlecan/HSPG2: Signaling role of domain IV in chondrocyte clustering with implications for Schwartz-Jampel Syndrome, J. Cell. Biochem 120 (2019) 2138–2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mongiat M, Sweeney SM, San Antonio JD, Fu J, Iozzo RV, Endorepellin, a novel inhibitor of angiogenesis derived from the C terminus of perlecan, J. Biol. Chem 278 (2003) 4238–4249. [DOI] [PubMed] [Google Scholar]
- 39.French MM, Gomes RR Jr., Timpl R, Hook M, Czymmek K, Farach-Carson MC, Carson DD, Chondrogenic activity of the heparan sulfate proteoglycan perlecan maps to the N-terminal domain I, J. Bone Miner. Res 17 (2002) 48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gomes RR Jr, Farach-Carson MC, Carson DD, Perlecan-stimulated nodules undergo chondrogenic maturation in response to rhBMP-2 treatment in vitro, Connect Tissue Res. 44 Suppl 1 (2003) 196–201. [PMC free article] [PubMed] [Google Scholar]
- 41.Perrimon N, Bernfield M, Specificities of heparan sulphate proteoglycans in developmental processes, Nature 404 (2000) 725–728. [DOI] [PubMed] [Google Scholar]
- 42.San Antonio JD, Winston BM, Tuan RS, Regulation of chondrogenesis by heparan sulfate and structurally related glycosaminoglycans, Dev. Biol 123 (1987) 17–24. [DOI] [PubMed] [Google Scholar]
- 43.Hoshiba T, Lu H, Yamada T, Kawazoe N, Tateishi T, Chen G, Effects of extracellular matrices derived from different cell sources on chondrocyte functions, Biotech. Prog 27 (2011) 788–795. [DOI] [PubMed] [Google Scholar]
- 44.Knox S, Melrose J, Whitelock J, Electrophoretic, biosensor, and bioactivity analyses of perlecans of different cellular origins, Proteomics 1 (2001) 1534–1530. [DOI] [PubMed] [Google Scholar]
- 45.Lord MS, Chuang CY, Melrose J, Davies MJ, Iozzo RV, Whitelock JM, The role of vascular-derived perlecan in modulating cell adhesion, proliferation and growth factor signaling, Matrix Biol. 35 (2014) 112–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shu CC, Jackson MT, Smith MM, Smith SM, Penm S, Lord MS, Whitelock JM, Little CB, Melrose J, Ablation of Perlecan Domain 1 Heparan Sulfate Reduces Progressive Cartilage Degradation, Synovitis, and Osteophyte Size in a Preclinical Model of Posttraumatic Osteoarthritis, Arthritis Rheum. 68 (2016) 868–879. [DOI] [PubMed] [Google Scholar]
- 47.Melrose J, Smith S, Whitelock J, Perlecan immunolocalizes to perichondrial vessels and canals in human fetal cartilaginous primordia in early vascular and matrix remodeling events associated with diarthrodial joint development, J. Histochem. Cytochem 52 (2004) 1405–1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chuang CY, Lord MS, Melrose J, Rees MD, Knox SM, Freeman C, Iozzo RV, Whitelock JM, Heparan Sulfate-Dependent Signaling of Fibroblast Growth Factor 18 by Chondrocyte-Derived Perlecan, Biochemistry 49 (2010) 5524–5532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Melrose J, Roughley P, Knox S, Smith S, Lord M, Whitelock J, The structure, location, and function of perlecan, a prominent pericellular proteoglycan of fetal, postnatal, and mature hyaline cartilages, J. Biol. Chem 281 (2006) 36905–36914. [DOI] [PubMed] [Google Scholar]
- 50.Jones BA, Pei M, Synovium-derived stem cells: a tissue-specific stem cell for cartilage engineering and regeneration, Tissue Eng. Part B Rev 18 (2012) 301–311. [DOI] [PubMed] [Google Scholar]
- 51.Pizzute T, Lynch K, Pei M, Impact of tissue-specific stem cells on lineage-specific differentiation: a focus on the musculoskeletal system, Stem Cell Rev. Rep 11 (2015) 119–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Arikawa-Hirasawa E, Watanabe H, Takami H, Hassell JR, Yamada Y, Perlecan is essential for cartilage and cephalic development, Nat. Genet 23 (1999) 354–358. [DOI] [PubMed] [Google Scholar]
- 53.Rodgers KD, Sasaki T, Aszodi A, Jacenko O, Reduced perlecan in mice results in chondrodysplasia resembling Schwartz-Jampel syndrome, Hum. Mol. Genet 16 (2007) 515–528. [DOI] [PubMed] [Google Scholar]
- 54.Costell M, Gustafsson E, Aszódi A, Mörgelin M, Bloch W, Hunziker E, Addicks K, Timpl R, Fässler R, Perlecan maintains the integrity of cartilage and some basement membranes, J. Cell Biol 147 (1999) 1109–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Handler M, Yurchenco PD, Iozzo RV, Developmental expression of perlecan during murine embryogenesis, Dev. Dyn 210 (1997) 130–145. [DOI] [PubMed] [Google Scholar]
- 56.Fujikawa K, Yokohama-Tamaki T, Morita T, Baba O, Shibata S, An In Situ Hybridization Study of Perlecan, DMP1, and MEPE in Developing Condylar Cartilage of the Fetal Mouse Mandible and Limb Bud Cartilage, Eur. J. Histochem 59 (2015) 211–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Roediger M, Kruegel J, Miosge N, Gersdorff N, Tissue distribution of perlecan domains III and V during embryonic and fetal human development, Histol. Histopathol 24 (2009) 859–868. [DOI] [PubMed] [Google Scholar]
- 58.French MM, Smith SE, Akanbi K, Sanford T, Hecht J, Farach-Carson MC, Carson DD, Expression of the heparan sulfate proteoglycan, perlecan, during mouse embryogenesis and perlecan chondrogenic activity in vitro, J. Cell Biol 145 (1999) 1103–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.West L, Govindraj P, Koob TJ, Hassell JR, Changes in perlecan during chondrocyte differentiation in the fetal bovine rib growth plate, J. Orthop. Res 24 (2006) 1317–1326. [DOI] [PubMed] [Google Scholar]
- 60.Melrose J, Smith S, Cake M, Read R, Whitelock J, Perlecan displays variable spatial and temporal immunolocalisation patterns in the articular and growth plate cartilages of the ovine stifle joint, Histochem. Cell Biol 123 (2005) 561–571. [DOI] [PubMed] [Google Scholar]
- 61.Clark CR, Ogden JA, Development of the menisci of the human knee joint. Morphological changes and their potential role in childhood meniscal injury, J. Bone Joint Surg. Am 65 (1983) 538–547. [PubMed] [Google Scholar]
- 62.Smith SM, Shu C, Melrose J, Comparative immunolocalisation of perlecan with collagen II and aggrecan in human foetal, newborn and adult ovine joint tissues demonstrates perlecan as an early developmental chondrogenic marker, Histochem. Cell Biol 134 (2010) 251–263. [DOI] [PubMed] [Google Scholar]
- 63.Melrose J, Smith S, Cake M, Read R, Whitelock J, Comparative spatial and temporal localisation of perlecan, aggrecan and type I, II and IV collagen in the ovine meniscus: an ageing study, Histochem. Cell Biol 124 (2005) 225–235. [DOI] [PubMed] [Google Scholar]
- 64.Melrose J, Smith S, Ghosh P, Whitelock J, Perlecan, the multidomain heparan sulfate proteoglycan of basement membranes, is also a prominent component of the cartilaginous primordia in the developing human fetal spine, J. Histochem. Cytochem 51 (2003) 1331–1341. [DOI] [PubMed] [Google Scholar]
- 65.Hayes AJ, Melrose J, 3D distribution of perlecan within intervertebral disc chondrons suggests novel regulatory roles for this multifunctional modular heparan sulphate proteoglycan, Eur. Cell Mater 41 (2021) 73–89. [DOI] [PubMed] [Google Scholar]
- 66.Hayes AJ, Melrose J, What are the potential roles of nuclear perlecan and other heparan sulphate proteoglycans in the normal and malignant phenotype, Int. J. Mol. Sci 22 (2021) 4415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shu C, Hughes C, Smith SM, Smith MM, Hayes A, Caterson B, Little CB, Melrose J, The ovine newborn and human foetal intervertebral disc contain perlecan and aggrecan variably substituted with native 7D4 CS sulphation motif: spatiotemporal immunolocalisation and co-distribution with Notch-1 in the human foetal disc, Glycoconj. J 30 (2013) 717–725. [DOI] [PubMed] [Google Scholar]
- 68.Shu C, Smith SS, Little CB, Melrose J, Comparative immunolocalisation of perlecan, heparan sulphate, fibroblast growth factor-18, and fibroblast growth factor receptor-3 and their prospective roles in chondrogenic and osteogenic development of the human foetal spine, Eur. Spine J 22 (2013) 1774–1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hayes AJ, Smith SM, Melrose J, Comparative immunolocalisation of fibrillin-1 and perlecan in the human foetal, and HS-deficient hspg2 exon 3 null mutant mouse intervertebral disc, Histochem. Cell Biol 139 (2013) 1–11. [DOI] [PubMed] [Google Scholar]
- 70.Iozzo RV, Cohen IR, Grässel S, Murdoch AD, The biology of perlecan: the multifaceted heparan sulphate proteoglycan of basement membranes and pericellular matrices, Biochem. J 302 (1994) 625–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hayes AJ, Smith SM, Gibson MA, Melrose J, Comparative Immunolocalization of the Elastin Fiber–Associated Proteins Fibrillin-1, LTBP-2, and MAGP-1 With Components of the Collagenous and Proteoglycan Matrix of the Fetal Human Intervertebral Disc, Spine 36 (2011) E1365–E1372. [DOI] [PubMed] [Google Scholar]
- 72.Hayes AJ, Gibson MA, Shu C, Melrose J, Confocal microscopy demonstrates association of LTBP-2 in fibrillin-1 microfibrils and colocalisation with perlecan in the disc cell pericellular matrix, Tissue Cell 46 (2014) 185–197. [DOI] [PubMed] [Google Scholar]
- 73.Hayes AJ, Shu CC, Lord MS, Little CB, Melrose J, Pericellular colocalisation and interactive properties of type VI collagen and perlecan in the intervertebral disc, Eur. Cells Mater 32 (2016) 40–57. [DOI] [PubMed] [Google Scholar]
- 74.Responte DJ, Lee JK, Hu JC, Athanasiou KA, Biomechanics-driven chondrogenesis: from embryo to adult, FASEB J. 26 (2012) 3614–3624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.McLeod MA, Wilusz RE, Guilak F, Depth-dependent anisotropy of the micromechanical properties of the extracellular and pericellular matrices of articular cartilage evaluated via atomic force microscopy, J. Biomech 46 (2013) 586–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mow VC, Guo XE, Mechano-electrochemical properties of articular cartilage: their inhomogeneities and anisotropies, Annu. Rev. Biomed. Eng 4 (2002) 175–209. [DOI] [PubMed] [Google Scholar]
- 77.Darling EM, Wilusz RE, Bolognesi MP, Zauscher S, Guilak F, Spatial mapping of the biomechanical properties of the pericellular matrix of articular cartilage measured in situ via atomic force microscopy, Biophys J. 98 (2010) 2848–2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Horikawa O, Nakajima H, Kikuchi T, Ichimura S, Yamada H, Fujikawa K, Toyama Y, Distribution of type VI collagen in chondrocyte microenvironment: study of chondrons isolated from human normal and degenerative articular cartilage and cultured chondrocytes, J Orthop. Sci 9 (2004) 29–36. [DOI] [PubMed] [Google Scholar]
- 79.Owida HA, De Las Heras Ruiz T, Dhillon A, Yang Y, Kuiper NJ, Co-culture of chondrons and mesenchymal stromal cells reduces the loss of collagen VI and improves extracellular matrix production, Histochem. Cell Biol 148 (2017) 625–638. [DOI] [PubMed] [Google Scholar]
- 80.Wilusz RE, DeFrate LE, Guilak F, Immunofluorescence-guided atomic force microscopy to measure the micromechanical properties of the pericellular matrix of porcine articular cartilage, J. R. Soc. Interface 9 (2012) 2997–3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Xu X, Li Z, Leng Y, Neu CP, Calve S, Knockdown of the pericellular matrix molecule perlecan lowers in situ cell and matrix stiffness in developing cartilage, Dev. Biol 418 (2016) 242–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chen C, Tambe DT, Deng L, Yang L, Biomechanical properties and mechanobiology of the articular chondrocyte, Am. J. Physiol. Cell Physiol 305 (2013) C1202–1208. [DOI] [PubMed] [Google Scholar]
- 83.Guilak F, Alexopoulos LG, Upton ML, Youn I, Choi JB, Cao L, Setton LA, Haider MA, The pericellular matrix as a transducer of biomechanical and biochemical signals in articular cartilage, Ann. New York Acad. Sci 1068 (2006) 498–512. [DOI] [PubMed] [Google Scholar]
- 84.Vincent TL, McLean CJ, Full LE, Peston D, Saklatvala J, FGF-2 is bound to perlecan in the pericellular matrix of articular cartilage, where it acts as a chondrocyte mechanotransducer, Osteoarthritis Cartilage 15 (2007) 752–763. [DOI] [PubMed] [Google Scholar]
- 85.Ocken AR, Ku MM, Kinzer-Ursem TL, Calve S, Perlecan Knockdown Significantly Alters Extracellular Matrix Composition and Organization During Cartilage Development, Mol. Cell. Proteomics 19 (2020) 1220–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kraft JJ, Jeong C, Novotny JE, Seacrist T, Chan G, Domzalski M, Turka CM, Richardson DW, Dodge GR, Effects of Hydrostatic Loading on a Self-Aggregating, Suspension Culture-Derived Cartilage Tissue Analog, Cartilage 2 (2011) 254–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.van Caam A, Cocca RA, Farran AJ, Mohanraj B, Meloni GR, Mauck RL, van der Kraan PM, Dodge GR, Perlecan expression is strongly reduced in aging cartilage but increased by physiological loading, Osteoarthritis Cartilage 22 (2014) S60. [Google Scholar]
- 88.Madej W, van Caam A, Davidson EN, Hannink G, Buma P, van der Kraan PM, Ageing is associated with reduction of mechanically-induced activation of Smad2/3P signaling in articular cartilage, Osteoarthritis Cartilage 24 (2016) 146–157. [DOI] [PubMed] [Google Scholar]
- 89.Melrose J, Hayes AJ, Whitelock JM, Little CB, Perlecan, the “jack of all trades” proteoglycan of cartilaginous weight-bearing connective tissues, BioEssays 30 (2008) 457–469. [DOI] [PubMed] [Google Scholar]
- 90.SundarRaj N, Fite D, Ledbetter S, Chakravarti S, Hassell JR, Perlecan is a component of cartilage matrix and promotes chondrocyte attachment, J. Cell Sci 108 (1995) 2663–2672. [DOI] [PubMed] [Google Scholar]
- 91.Itoh N, Ornitz DM, Fibroblast growth factors: from molecular evolution to roles in development, metabolism and disease, J. Biochem 149 (2011) 121–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ornitz DM, Itoh N, Fibroblast growth factors, Genome Biol. 2 (2001) REVIEWS3005.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ellman MB, Yan D, Ahmadinia K, Chen D, An HS, Im HJ, Fibroblast growth factor control of cartilage homeostasis, J. Cell. Biochem 114 (2013) 735–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Krejci P, Krakow D, Mekikian PB, Wilcox WR, Fibroblast Growth Factors 1, 2, 17, and 19 Are the Predominant FGF Ligands Expressed in Human Fetal Growth Plate Cartilage, Pediatr. Res 61 (2007) 267–272. [DOI] [PubMed] [Google Scholar]
- 95.Chia SL, Sawaji Y, Burleigh A, Mclean C, Inglis J, Saklatvala J, Vincent T, Fibroblast growth factor 2 is an intrinsic chondroprotective agent that suppresses ADAMTS-5 and delays cartilage degradation in murine osteoarthritis, Arthritis Rheum. 60 (2014) 2019–2027. [DOI] [PubMed] [Google Scholar]
- 96.Knox S, Merry C, Stringer S, Melrose J, Whitelock J, Not all perlecans are created equal - Interactions with fibroblast growth factor (FGF) 2 and FGF receptors, J. Biol. Chem 277 (2002) 14657–14665. [DOI] [PubMed] [Google Scholar]
- 97.Govindraj P, West L, Smith S, Hassell JR, Modulation of FGF-2 binding to chondrocytes from the developing growth plate by perlecan, Matrix Biol. 25 (2006) 232–239. [DOI] [PubMed] [Google Scholar]
- 98.Smith SM, West LA, Govindraj P, Zhang X, Ornitz DM, Hassell JR, Heparan and chondroitin sulfate on growth plate perlecan mediate binding and delivery of FGF-2 to FGF receptors, Matrix Biol. 26 (2007) 175–184. [DOI] [PubMed] [Google Scholar]
- 99.Liu Z, Lavine KJ, Hung IH, Ornitz DM, FGF18 is required for early chondrocyte proliferation, hypertrophy and vascular invasion of the growth plate, Dev. Biol 302 (2007) 80–91. [DOI] [PubMed] [Google Scholar]
- 100.Davidson D, Blanc A, Filion D, Wang H, Plut P, Pfeffer G, Buschmann MD, Henderson JE, Fibroblast growth factor (FGF) 18 signals through FGF receptor 3 to promote chondrogenesis, J. Biol. Chem 280 (2005) 20509–20515. [DOI] [PubMed] [Google Scholar]
- 101.Liu Z, Xu J, Colvin JS, Ornitz DM, Coordination of chondrogenesis and osteogenesis by fibroblast growth factor 18, Genes Dev. 16 (2002) 859–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chuang CY, Shahin K, Lord MS, Melrose J, Doran PM, Whitelock JM, The cartilage matrix molecule components produced by human foetal cartilage rudiment cells within scaffolds and the role of exogenous growth factors, Biomaterials 33 (2012) 4078–4088. [DOI] [PubMed] [Google Scholar]
- 103.Smith ML, West LA, Hassell JR, The core protein of growth plate perlecan binds FGF-18 and alters its mitogenic effect on chondrocytes, Arch. Biochem. Biophys 468 (2007) 244–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Correa D, Somoza RA, Lin P, Greenberg S, Rom E, Duesler L, Welter JF, Yayon A, Caplan AI, Sequential exposure to fibroblast growth factors (FGF) 2, 9 and 18 enhances hMSC chondrogenic differentiation, Osteoarthritis Cartilage 23 (2015) 443–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hall BK, Miyake T, All for one and one for all: condensations and the initiation of skeletal development, BioEssays 22 (2000) 138–147. [DOI] [PubMed] [Google Scholar]
- 106.Chijimatsu R, Saito T, Mechanisms of synovial joint and articular cartilage development, Cell Mol. Life Sci 76 (2019) 3939–3952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Goldring MB, Tsuchimochi K, Ijiri K, The control of chondrogenesis, J. Cell. Biochem 97 (2006) 33–44. [DOI] [PubMed] [Google Scholar]
- 108.Gomes RR Jr, Joshi SS, Farach-Carson MC, Carson DD, Ribozyme-mediated perlecan knockdown impairs chondrogenic differentiation of C3H10T1/2 fibroblasts, Differentiation 74 (2006) 53–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Seghatoleslami MR, Roman-Blas JA, Rainville AM, Modaressi R, Danielson KG, Tuan RS, Progression of chondrogenesis in C3H10T1/2 cells is associated with prolonged and tight regulation of ERK1/2, J. Cell. Biochem 88 (2003) 1129–1144. [DOI] [PubMed] [Google Scholar]
- 110.Kirn-Safran CB, Gomes RR, Brown AJ, Carson DD, Heparan Sulfate Proteoglycans: Coordinators of Multiple Signaling Pathways during Chondrogenesis, Birth Defects Res. C Embryo Today 72 (2004) 69–88. [DOI] [PubMed] [Google Scholar]
- 111.Jha AK, Yang W, Kirn-Safran CB, Farach-Carson MC, Jia X, Perlecan domain I-conjugated, hyaluronic acid-based hydrogel particles for enhanced chondrogenic differentiation via BMP-2 release, Biomaterials 30 (2009) 6964–6975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Decarlo AA, Belousova M, Ellis AL, Petersen D, Grenett H, Hardigan P, O’Grady R, Lord M, Whitelock JM, Perlecan domain 1 recombinant proteoglycan augments BMP-2 activity and osteogenesis, BMC Biotechnol. 12 (2012) 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Srinivasan PP, Mccoy SY, Jha AK, Yang W, Jia X, Farach-Carson MC, Kirn-Safran CB, Injectable perlecan domain 1-hyaluronan microgels potentiate the cartilage repair effect of BMP2 in a murine model of early osteoarthritis, Biomed. Mater 7 (2012) 024109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Takada T, Katagiri T, Ifuku M, Morimura N, Kobayashi M, Hasegawa K, Ogamo A, Kamijo R, Sulfated Polysaccharides Enhance the Biological Activities of Bone Morphogenetic Proteins, J. Biol. Chem 278 (2003) 43229–43235. [DOI] [PubMed] [Google Scholar]
- 115.Nakanishi T, Kimura Y, Tamura T, Ichikawa H, Yamaai Y, Sugimoto T, Takigawa M, Cloning of a mRNA preferentially expressed in chondrocytes by differential display-PCR from a human chondrocytic cell line that is identical with connective tissue growth factor (CTGF) mRNA, Biochem. Biophys. Res. Comm 234 (1997) 206–210. [DOI] [PubMed] [Google Scholar]
- 116.Brigstock DR, The CCN family: a new stimulus package, J Endocrinol. 178 (2003) 169–175. [DOI] [PubMed] [Google Scholar]
- 117.de Winter P, Leoni P, Abraham D, Connective tissue growth factor: structure-function relationships of a mosaic, multifunctional protein, Growth factors 26 (2008) 80–91. [DOI] [PubMed] [Google Scholar]
- 118.Nakanishi T, Nishida T, Shimo T, Kobayashi K, Kubo T, Tamatani T, Tezuka K, Takigawa M, Effects of CTGF/Hcs24, a product of a hypertrophic chondrocyte-specific gene, on the proliferation and differentiation of chondrocytes in culture, Endocrinology 141 (2000) 264–273. [DOI] [PubMed] [Google Scholar]
- 119.Perbal B, CCN proteins: multifunctional signalling regulators, Lancet 363 (2004) 62–64. [DOI] [PubMed] [Google Scholar]
- 120.Nishida T, Kubota S, Nakanishi T, Kuboki T, Yosimichi G, Kondo S, Takigawa M, CTGF/Hcs24, a hypertrophic chondrocyte-specific gene product, stimulates proliferation and differentiation, but not hypertrophy of cultured articular chondrocytes, J. Cell Physiol 192 (2002) 55–63. [DOI] [PubMed] [Google Scholar]
- 121.Arnott JA, Lambi AG, Mundy C, Hendesi H, Pixley RA, Owen TA, Safadi FF, Popoff SN, The role of connective tissue growth factor (CTGF/CCN2) in skeletogenesis, Crit. Rev. Eukaryot. Gene Expr 21 (2011) 43–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Fitzgerald J, Su TT, Bateman JF, WARP is a new member of the von Willebrand factor A-domain superfamily of extracellular matrix proteins, FEBS letters 517 (2002) 61–66. [DOI] [PubMed] [Google Scholar]
- 123.Hansen U, Allen JM, White R, Moscibrocki C, Bruckner P, Bateman JF, Fitzgerald J, WARP Interacts with Collagen VI-Containing Microfibrils in the Pericellular Matrix of Human Chondrocytes, PloS One 7 (2012) e52793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Allen JM, Brachvogel B, Farlie PG, Fitzgerald J, Bateman JF, The extracellular matrix protein WARP is a novel component of a distinct subset of basement membranes, Matrix Biol. 27 (2008) 295–305. [DOI] [PubMed] [Google Scholar]
- 125.Allen JM, Zamurs L, Brachvogel B, Schlotzer-Schrehardt U, Hansen U, Lamande SR, Rowley L, Fitzgerald J, Bateman JF, Mice lacking the extracellular matrix protein WARP develop normally but have compromised peripheral nerve structure and function, J. Biol. Chem 284 (2009) 12020–12030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Fitzgerald J, WARP: A Unique Extracellular Matrix Component of Cartilage, Muscle, and Endothelial Cell Basement Membranes, Anat. Rec 303 (2020) 1619–1623. [DOI] [PubMed] [Google Scholar]
- 127.Gubbiotti MA, Neill T, Iozzo RV, A current view of perlecan in physiology and pathology: A mosaic of functions, Matrix Biol. 57–58 (2017) 285–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hayes AJ, Smith SM, Caterson B, Melrose J, Concise Review: Stem/Progenitor Cell Proteoglycans Decorated with 7-D-4, 4-C-3, and 3-B-3(−) Chondroitin Sulfate Motifs Are Morphogenetic Markers of Tissue Development, Stem Cells 36 (2018) 1475–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Knox S, Fosang AJ, Last K, Melrose J, Whitelock J, Perlecan from human epithelial cells is a hybrid heparan/chondroitin/keratan sulfate proteoglycan, FEBS Lett. 579 (2005) 5019–5023. [DOI] [PubMed] [Google Scholar]
- 130.Melrose J, Isaacs MD, Smith SM, Hughes CE, Little CB, Caterson B, Hayes AJ, Chondroitin sulphate and heparan sulphate sulphation motifs and their proteoglycans are involved in articular cartilage formation during human foetal knee joint development, Histochem. Cell Biol 138 (2012) 461–475. [DOI] [PubMed] [Google Scholar]
- 131.Smith MM, Melrose J, Perlecan delineates stem cell niches in human foetal hip, knee and elbow cartilage rudiments and has potential roles in the regulation of stem cell differentiation, J. Stem Cell Res. Dev. Ther 3 (2016) 9–16. [Google Scholar]
- 132.Hayes AJ, Tudor D, Nowell MA, Caterson B, Hughes CE, Chondroitin sulfate sulfation motifs as putative biomarkers for isolation of articular cartilage progenitor cells, J. Histochem. Cytochem 56 (2008) 125–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Chong KW, Chanalaris A, Burleigh A, Jin H, Watt FE, Saklatvala J, Vincent TL, Fibroblast growth factor 2 drives changes in gene expression following injury to murine cartilage in vitro and in vivo, Arthritis Rheum. 65 (2013) 2346–2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Vincent T, Hermansson M, Bolton M, Wait R, Saklatvala J, Basic FGF mediates an immediate response of articular cartilage to mechanical injury, Proc. Nat. Acad. Sci. U.S.A 99 (2002) 8259–8264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Im HJ, Muddasani P, Natarajan V, Schmid TM, Block JA, Davis F, van Wijnen AJ, Loeser RF, Basic fibroblast growth factor stimulates matrix metalloproteinase-13 via the molecular cross-talk between the mitogen-activated protein kinases and protein kinase Cdelta pathways in human adult articular chondrocytes, J. Biol. Chem 282 (2007) 11110–11121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Yan D, Chen D, Cool SM, van Wijnen AJ, Mikecz K, Murphy G, Im HJ, Fibroblast growth factor receptor 1 is principally responsible for fibroblast growth factor 2-induced catabolic activities in human articular chondrocytes, Arthritis Res. Ther 13 (2011) R130. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 137.Yan D, Chen D, Im HJ, Fibroblast growth factor-2 promotes catabolism via FGFR1-Ras-Raf-MEK1/2-ERK1/2 axis that coordinates with the PKCdelta pathway in human articular chondrocytes, J. Cell. Biochem 113 (2012) 2856–2865. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 138.Lanner F, Rossant J, The role of FGF/Erk signaling in pluripotent cells, Development 137 (2010) 3351–3360. [DOI] [PubMed] [Google Scholar]
- 139.Vincent TL, Hermansson MA, Hansen UN, Amis AA, Saklatvala J, Basic fibroblast growth factor mediates transduction of mechanical signals when articular cartilage is loaded, Arthritis Rheum. 50 (2004) 526–533. [DOI] [PubMed] [Google Scholar]
- 140.Salazar VS, Gamer LW, Rosen V, BMP signalling in skeletal development, disease and repair, Nat. Rev. Endocrinol 12 (2016) 203–221. [DOI] [PubMed] [Google Scholar]
- 141.Yang W, Gomes RR, Brown AJ, Burdett AR, Alicknavitch M, Farach-Carson MC, Carson DD, Chondrogenic Differentiation on Perlecan Domain I, Collagen II, and Bone Morphogenetic Protein-2–Based Matrices, Tissue Eng. 12 (2006) 2009–2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ruppert R, Hoffmann E, Sebald W, Human bone morphogenetic protein 2 contains a heparin-binding site which modifies its biological activity, Eur J Biochem. 237 (1996) 295–302. [DOI] [PubMed] [Google Scholar]
- 143.Jiao X, Billings PC, O’Connell MP, Kaplan FS, Shore EM, Glaser DL, Heparan sulfate proteoglycans (HSPGs) modulate BMP2 osteogenic bioactivity in C2C12 cells, J. Biol. Chem 282 (2007) 1080–1086. [DOI] [PubMed] [Google Scholar]
- 144.Wu M, Chen G, Li YP, TGF-β and BMP signaling in osteoblast, skeletal development, and bone formation, homeostasis and disease, Bone Res. 4 (2016) 16009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Yoon BS, Lyons KM, Multiple functions of BMPs in chondrogenesis, J. Cell Biochem 93 (2004) 93–103. [DOI] [PubMed] [Google Scholar]
- 146.Lee KS, Hong SH, Bae SC, Both the Smad and p38 MAPK pathways play a crucial role in Runx2 expression following induction by transforming growth factor-beta and bone morphogenetic protein, Oncogene 21 (2002) 7156–7163. [DOI] [PubMed] [Google Scholar]
- 147.Thielen NGM, van der Kraan PM, van Caam APM, TGFβ/BMP Signaling Pathway in Cartilage Homeostasis, Cells 8 (2019) 969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Yamaguchi K, Shirakabe K, Shibuya H, Irie K, Oishi I, Ueno N, Taniguchi T, Nishida E, Matsumoto K, Identification of a member of the MAPKKK family as a potential mediator of TGF-beta signal transduction, Science 270 (1995) 2008–2011. [DOI] [PubMed] [Google Scholar]
- 149.Gao L, Sheu TJ, Dong Y, Hoak DM, Zuscik MJ, Schwarz EM, Hilton MJ, O’Keefe RJ, Jonason JH, TAK1 regulates SOX9 expression in chondrocytes and is essential for postnatal development of the growth plate and articular cartilages, J. Cell Sci 126 (2013) 5704–5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Greenblatt MB, Shim JH, Zou W, Sitara D, Schweitzer M, Hu D, Lotinun S, Sano Y, Baron R, Park JM, et al. The p38 MAPK pathway is essential for skeletogenesis and bone homeostasis in mice, J Clin. Invest 120 (2010) 2457–2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kubota S, Takigawa M, The role of CCN2 in cartilage and bone development. Journal of cell communication and signaling, 5 (2011) 209–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Segarini PR, Nesbitt JE, Li D, Hays LG, Yates JR 3rd, Carmichael DF, The low density lipoprotein receptor-related protein/alpha2-macroglobulin receptor is a receptor for connective tissue growth factor, J. Biol. Chem 276 (2001) 40659–40667. [DOI] [PubMed] [Google Scholar]
- 153.Tang X, Muhammad H, McLean C, Miotla-Zarebska J, Fleming J, Didangelos A, Önnerfjord P, Leask A, Saklatvala J, Vincent TL, Connective tissue growth factor contributes to joint homeostasis and osteoarthritis severity by controlling the matrix sequestration and activation of latent TGFβ, Ann. Rheum. Dis 77 (2018) 1372–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Yosimichi G, Kubota S, Nishida T, Kondo S, Yanagita T, Nakao K, Takano-Yamamoto T, Takigawa M, Roles of PKC, PI3K and JNK in multiple transduction of CCN2/CTGF signals in chondrocytes, Bone 38 (2006) 853–863. [DOI] [PubMed] [Google Scholar]
- 155.Yosimichi G, Nakanishi T, Nishida T, Hattori T, Takano-Yamamoto T, Takigawa M, CTGF/Hcs24 induces chondrocyte differentiation through a p38 mitogen-activated protein kinase (p38MAPK), and proliferation through a p44/42 MAPK/extracellular-signal regulated kinase (ERK), Eur. J. Biochem 268 (2001) 6058–6065. [DOI] [PubMed] [Google Scholar]
- 156.Panda DK, Miao D, Lefebvre V, Hendy GN, Goltzman D, The transcription factor SOX9 regulates cell cycle and differentiation genes in chondrocytic CFK2 cells, J. Biol. Chem 276 (2001) 41229–41236. [DOI] [PubMed] [Google Scholar]
- 157.James CG, Woods A, Underhill TM, Beier F, The transcription factor ATF3 is upregulated during chondrocyte differentiation and represses cyclin D1 and A gene transcription, BMC Mol. Biol 7 (2006) 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Gustafsson E, Almonte-Becerril M, Bloch W, Costell M, Perlecan Maintains microvessel integrity in vivo and modulates their formation in vitro, PloS One 8 (2013) e53715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Lord MS, Ellis AL, Farrugia BL, Whitelock JM, Grenett H, Li C, O’Grady RL, DeCarlo AA, Perlecan and vascular endothelial growth factor-encoding DNA-loaded chitosan scaffolds promote angiogenesis and wound healing, J Control Release 250 (2017) 48–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Carlevaro MF, Cermelli S, Cancedda R, Cancedda FD, Vascular endothelial growth factor (VEGF) in cartilage neovascularization and chondrocyte differentiation: Auto-paracrine role during endochondral bone formation, J. Cell Sci 113 (2000) 59–69. [DOI] [PubMed] [Google Scholar]
- 161.Duan X, Murata Y, Liu Y, Nicolae C, Olsen BR, Berendsen AD, Vegfa regulates perichondrial vascularity and osteoblast differentiation in bone development, Development 142 (2015) 1984–1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Zelzer E, Mamluk R, Ferrara N, Johnson RS, Schipani E, Olsen BR, VEGFA is necessary for chondrocyte survival during bone development, Development 131 (2004) 2161–2171. [DOI] [PubMed] [Google Scholar]
- 163.Goyal A, Pal N, Concannon M, Paul M, Doran M, Poluzzi C, Sekiguchi K, Whitelock JM, Neill T, Iozzo RV, Endorepellin, the angiostatic module of perlecan, interacts with both the α2β1 integrin and vascular endothelial growth factor receptor 2 (VEGFR2): a dual receptor antagonism, J. Biol. Chem 286 (2011) 25947–25962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Muthusamy A, Cooper CR, Gomes RR Jr, Soluble perlecan domain I enhances vascular endothelial growth factor-165 activity and receptor phosphorylation in human bone marrow endothelial cells. BMC Biochem. 11 (2010) 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Willis CD, Poluzzi C, Mongiat M, Iozzo RV, Endorepellin laminin-like globular 1/2 domains bind Ig3-5 of vascular endothelial growth factor (VEGF) receptor 2 and block pro-angiogenic signaling by VEGFA in endothelial cells, FEBS J. 280 (2013) 2271–2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Neufeld G, Cohen T, Gengrinovitch S, Poltorak Z, Vascular endothelial growth factor (VEGF) and its receptors, FASEB J. 13 (1999) 9–22. [PubMed] [Google Scholar]
- 167.D’Souza S, Yang W, Marchetti D, Muir C, Farach-Carson MC, Carson DD, HIP/RPL29 antagonizes VEGF and FGF2 stimulated angiogenesis by interfering with HS-dependent responses, J. Cell. Biochem 105 (2008) 1183–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Zoeller JJ, Whitelock JM, Iozzo RV, Perlecan regulates developmental angiogenesis by modulating the VEGF-VEGFR2 axis, Matrix Biol. 28 (2009) 284–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Ishijima M, Suzuki N, Hozumi K, Matsunobu T, Kosaki K, Kaneko H, Hassell JR, Arikawa-Hirasawa E, Yamada Y, Perlecan modulates VEGF signaling and is essential for vascularization in endochondral bone formation, Matrix Biol. 31 (2012) 234–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kapoor A, Chen CG, Iozzo RV, Endorepellin evokes an angiostatic stress signaling cascade in endothelial cells, J. Biol. Chem 295 (2020) 6344–6356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Nyström A, Shaik ZP, Gullberg D, Krieg T, Eckes B, Zent R, Pozzi A, Iozzo RV, Role of tyrosine phosphatase SHP-1 in the mechanism of endorepellin angiostatic activity, Blood 114 (2009) 4897–4906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Tesche F, Miosge N, Perlecan in late stages of osteoarthritis of the human knee joint, Osteoarthritis Cartilage 12 (2004) 852–862. [DOI] [PubMed] [Google Scholar]
- 173.Löfgren M, Svala E, Lindahl A, Skiöldebrand E, Ekman S, Time-dependent changes in gene expression induced in vitro by interleukin-1β in equine articular cartilage, Res. Vet. Sci 118 (2018) 466–476. [DOI] [PubMed] [Google Scholar]
- 174.Sherwood J, Bertrand J, Nalesso G, Poulet B, Pitsillides A, Brandolini L, Karystinou A, De Bari C, Luyten FP, Pitzalis C, et al. A homeostatic function of CXCR2 signalling in articular cartilage, Ann. Rheum. Dis 74 (2015) 2207–2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Guilak F, Nims RJ, Dicks A, Wu CL, Meulenbelt I, Osteoarthritis as a disease of the cartilage pericellular matrix, Matrix Biol. 71–72 (2018) 40–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Chen J, Wang Y, Chen C, Lian C, Zhou T, Gao B, Wu Z, Xu C, Exogenous Heparan Sulfate Enhances the TGF-beta3-Induced Chondrogenesis in Human Mesenchymal Stem Cells by Activating TGF-beta/Smad Signaling, Stem cells Inter 2016 (2016) 1520136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Sadatsuki R, Kaneko H, Futami I, Hada S, Culley KL, Otero M, Dragomir C, Kinoshita M, Goldring MB, Yamada Y, Role of perlecan in chondrogenic, osteogenic and adipogenic differentiation of synovial mesenchymal calls, Osteoarthritis Cartilage 22 (2014) S439. [Google Scholar]
- 178.Sadatsuki R, Kaneko H, Kinoshita M, Futami I, Nonaka R, Culley KL, Otero M, Hada S, Goldring MB, Yamada Y, et al. Perlecan is required for the chondrogenic differentiation of synovial mesenchymal cells through regulation of Sox9 gene expression, J. Orthop. Res 35 (2017) 837–846. [DOI] [PubMed] [Google Scholar]
- 179.Mayuko K, Kaneko H, Sadatsuki R, Futami I, Hada S, Arita H, Shiozawa J, Hirasawa-Arikawa E, Yamada Y, Kaneko K, Perlecan regulates chondrogenic differentiation from synovial mesenchymal cells via Smad and MAPK signaling pathways, Osteoarthritis Cartilage 24 (2016) S177. [Google Scholar]
- 180.Smith SM, Melrose J, Type XI collagen-perlecan-HS interactions stabilise the pericellular matrix of annulus fibrosus cells and chondrocytes providing matrix stabilisation and homeostasis, J. Mol. Histol 50 (2019) 285–294. [DOI] [PubMed] [Google Scholar]
- 181.Hayes AJ, Lord MS, Smith SM, Smith MM, Whitelock JM, Weiss AS, Melrose J, Colocalization in vivo and association in vitro of perlecan and elastin, Histochem. Cell Biol 136 (2011) 437–454. [DOI] [PubMed] [Google Scholar]
- 182.Johnstone B, Alini M, Cucchiarini M, Dodge GR, Eglin D, Guilak F, Madry H, Mata A, Mauck RL, Semino CE, et al. Tissue engineering for articular cartilage repair--the state of the art, Eur. Cells Mater 25 (2013) 248–267. [DOI] [PubMed] [Google Scholar]
- 183.Farach-Carson MC, Carson DD, Perlecan—a multifunctional extracellular proteoglycan scaffold, Glycobiology 17 (2007) 897–905. [DOI] [PubMed] [Google Scholar]
- 184.Casper CL, Yang W, Farach-Carson MC, Rabolt JF, Coating Electrospun Collagen and Gelatin Fibers with Perlecan Domain I for Increased Growth Factor Binding, Biomacromolecules 8 (2007) 1116–1123. [DOI] [PubMed] [Google Scholar]
- 185.Garcia J, McCarthy HS, Kuiper JH, Melrose J, Roberts S, Perlecan in the natural and cell therapy repair of human adult articular cartilage: can modifications in this proteoglycan be a novel therapeutic approach? Biomolecules 11 (2021) 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Pei M, Environmental preconditioning rejuvenates adult stem cells’ proliferation and chondrogenic potential, Biomaterials 117 (2017) 10–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Pei M, Li JT, Shoukry M, Zhang Y, A Review of Decellularized Stem Cell Matrix: a Novel Cell Expansion System for Cartilage Tissue Engineering, Eur. Cell Mater 22 (2011) 333–343. [DOI] [PubMed] [Google Scholar]
- 188.Sun Y, Yan LQ, Chen S, Pei M, Functionality of decellularized matrix in cartilage regeneration: a comparison of tissue versus cell sources, Acta Biomater. 74 (2018) 56–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Wang Y, Fu Y, Yan Z, Zhang XB, Pei M, Impact of Fibronectin Knockout on Proliferation and Differentiation of Human Infrapatellar Fat Pad-Derived Stem Cells, Front. Bioeng. Biotechnol 7 (2019) 321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Bix GJ, Perlecan domain V therapy for stroke: a beacon of hope? ACS Chem. Neurosci 4 (2013) 370–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Lee B, Clarke D, Al Ahmad A, Kahle M, Parham C, Auckland L, Shaw C, Fidanboylu M, Orr AW, Ogunshola O, Fertala A, Thomas SA, Bix GJ, Perlecan domain V is neuroprotective and proangiogenic following ischemic stroke in rodents, J. Clin. Invest 121 (2011) 3005–3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Kaneko H, Ishijima M, Futami I, Tomikawa-Ichikawa N, Kosaki K, Sadatsuki R, Yamada Y, Kurosawa H, Kaneko K, Arikawa-Hirasawa E, Synovial perlecan is required for osteophyte formation in knee osteoarthritis, Matrix Biol. 32 (2013) 178–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Nakamura R, Nakamura F, Fukunaga S, Disruption of endogenous perlecan function improves differentiation of rat articular chondrocytes in vitro, Anim. Sci. J 86 (2015) 449–458. [DOI] [PubMed] [Google Scholar]
- 194.Whitelock JM, Melrose J, Iozzo RV, Diverse cell signaling events modulated by perlecan, Biochemistry 47 (2008) 11174–11183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Bengtsson E, Mörgelin M, Sasaki T, Timpl R, Heinegård D, Aspberg A, The leucine-rich repeat protein PRELP binds perlecan and collagens and may function as a basement membrane anchor, J. Biol. Chem 277 (2002) 15061–15068. [DOI] [PubMed] [Google Scholar]
- 196.Ettner N, Göhring W, Sasaki T, Mann K, Timpl R, The N-terminal globular domain of the laminin alpha1 chain binds to alpha1beta1 and alpha2beta1 integrins and to the heparan sulfate-containing domains of perlecan, FEBS letters 430 (1998) 217–221. [DOI] [PubMed] [Google Scholar]
- 197.Iozzo RV, San Antonio JD, Heparan sulfate proteoglycans: heavy hitters in the angiogenesis arena, J. Clin. Invest 108 (2001) 349–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Xu Y, Liu YJ, Yu Q, Angiopoietin-3 is tethered on the cell surface via heparan sulfate proteoglycans, J. Biol. Chem 279 (2004) 41179–41188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Göhring W, Sasaki T, Heldin CH, Timpl R, Mapping of the binding of platelet-derived growth factor to distinct domains of the basement membrane proteins BM-40 and perlecan and distinction from the BM-40 collagen-binding epitope, Eur. J. Biochem 255 (1998) 60–66. [DOI] [PubMed] [Google Scholar]
- 200.Hopf M, Gohring W, Kohfeldt E, Yamada Y, Timpl R, Recombinant domain IV of perlecan binds to nidogens, laminin-nidogen complex, fibronectin, fibulin-2 and heparin, Eur. J Biochem 259 (1999) 917–925. [DOI] [PubMed] [Google Scholar]
- 201.Hopf M, Göhring W, Mann K, Timpl R, Mapping of binding sites for nidogens, fibulin-2, fibronectin and heparin to different IG modules of perlecan, J. Mol. Biol 311 (2001) 529–541. [DOI] [PubMed] [Google Scholar]
- 202.Brown JC, Sasaki T, Gohring W, Yamada Y, Timpl R, The C-terminal domain V of perlecan promotes beta1 integrin-mediated cell adhesion, binds heparin, nidogen and fibulin-2 and can be modified by glycosaminoglycans, Eur. J. Biochem 250 (1997) 39–46. [DOI] [PubMed] [Google Scholar]
- 203.Mongiat M, Fu J, Oldershaw R, Greenhalgh R, Gown AM, Iozzo RV, Perlecan protein core interacts with extracellular matrix protein 1 (ECM1), a glycoprotein involved in bone formation and angiogenesis, J. Biol. Chem 278 (2003) 17491–17499. [DOI] [PubMed] [Google Scholar]
- 204.Yang WD, Gomes RR Jr, Alicknavitch M, Farach-Carson MC, Carson DD, Perlecan domain I promotes fibroblast growth factor 2 delivery in collagen I fibril scaffolds, Tissue Eng. 11 (2005) 76–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Hartman O, Zhang C, Adams EL, Farach-Carson MC, Petrelli NJ, Chase BD, Rabolt JF, Biofunctionalization of electrospun PCL-based scaffolds with perlecan domain IV peptide to create a 3D pharmacokinetic cancer model, Biomaterials 31 (2010) 5700–5718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Rnjak-Kovacina J, Tang FY, Whitelock JM, Lord MS, Silk biomaterials functionalized with recombinant domain V of human perlecan modulate endothelial cell and platelet interactions for vascular applications, Colloids Surf. B Biointerfaces 148 (2016) 130–138. [DOI] [PubMed] [Google Scholar]
- 207.Chiu YC, Fong EL, Grindel BJ, Kasper FK, Harrington DA, Farach-Carson MC, Sustained delivery of recombinant human bone morphogenetic protein-2 from perlecan domain I - functionalized electrospun poly (epsilon-caprolactone) scaffolds for bone regeneration, J. Exp. Orthop 3 (2016) 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Li KJ, Yang SJ, Li K, Wei YT, Ge J, Research on the effect of biological glue on augmenting the viscosity of tissue engineering retinal nerve scaffolds, Zhonghua Yan Ke Za Zhi 54 (2018) 277–282. [DOI] [PubMed] [Google Scholar]


