Figure 7.
Mis12C regulation of anaphase kinetochores
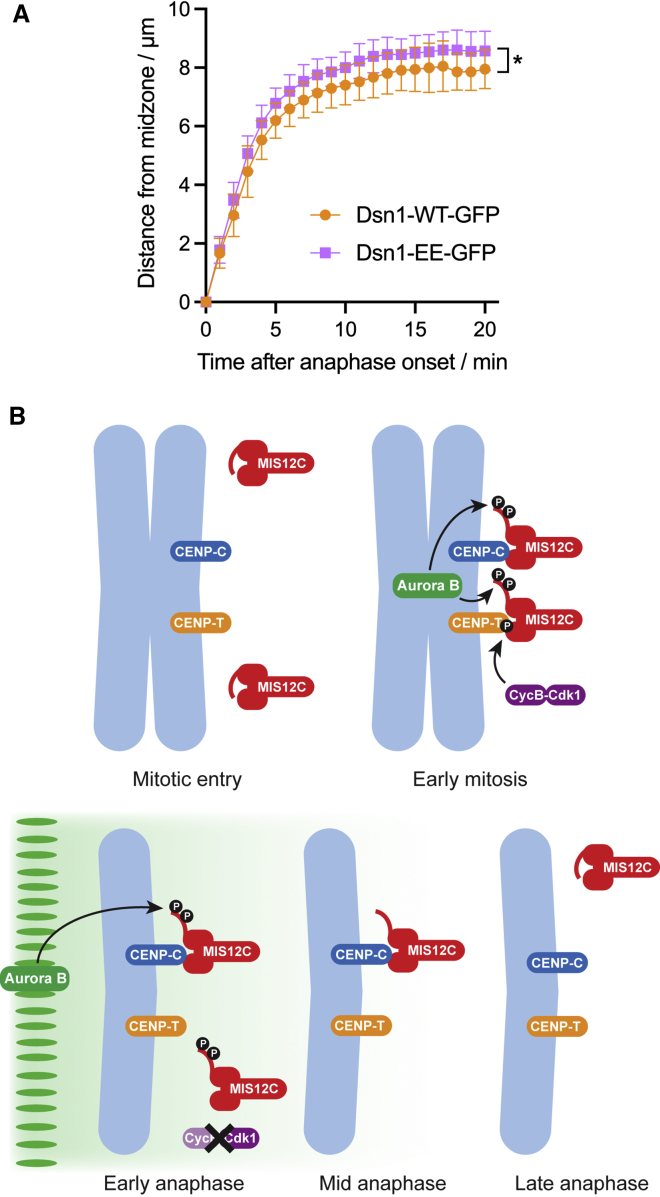
(A) Chromosome movements in living HeLa cells expressing Dsn1-WT-GFP or Dsn1-EE-GFP imaged at 1-min intervals. For Dsn1-WT-GFP, n = 10; and for Dsn1-EE-GFP, n = 11. Data are represented as mean ± SD. ∗p = 0.028 by two-way ANOVA.
(B) Model. In early mitosis, phosphorylation at S100/S109 by Aurora B (green) displaces the basic region of Dsn1 and allows Mis12C (red) binding to CENP-C (blue) and CENP-T (orange). Phosphorylation of CENP-T by Cyclin B-Cdk1 (purple) is also required for Mis12C to bind CENP-T. In early anaphase, Cyclin B degradation and loss of Cdk1-dependent CENP-T phosphorylation releases Mis12C from CENP-T, while Aurora B gradient activity can prolong phosphorylation of Dsn1 S100/S109, allowing Mis12C retention on CENP-C. As chromosomes move away from the central spindle, declining Aurora B activity allows dephosphorylation of Dsn1 S100/S109, although full release of Mis12C is delayed because phosphorylation is not directly required for CENP-C binding. By late anaphase, Dsn1 returns to its autoinhibited conformation, and all Mis12C is released.