Figure 1. Schematic representation of insulin signaling.
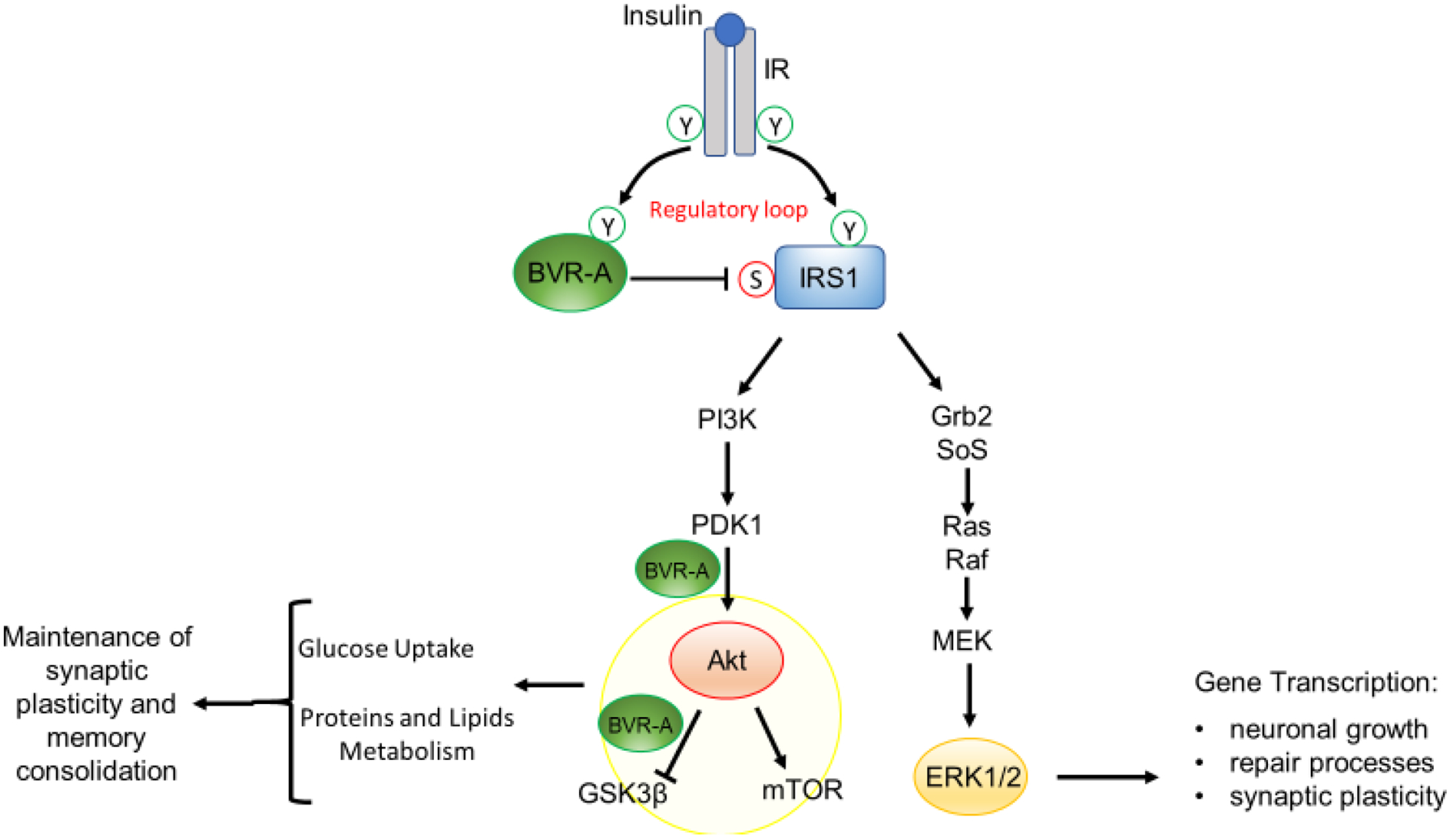
Under physiological conditions, the activation of insulin signaling requires the binding of insulin to the insulin receptor (IR), which auto-phosphorylates on Tyr residues (Y, e.g., Tyr1158/1162/1163) and promotes the receptor tyrosine kinase-mediated phosphorylation of its substrate (IRS1) on specific Tyr residues (e.g., 632). In parallel, IR phosphorylates BVR-A on specific Tyr residues and activates BVR-A to function as Ser/Thr/Tyr kinase. Then, as part of a regulatory loop, BVR-A phosphorylates IRS1 on inhibitory Ser residues (S, e.g., Ser307/312/616) to avoid IRS1 aberrant activation in response to IR. Once activated, IRS1 works as a scaffold protein, driving the activation of the two main arms of the insulin signaling: (1) the Ras/Raf/MAPK pathway (ERK1/2) mainly involved in gene transcription; and (2) the PI3K/Akt axis that is critical for glucose uptake as well as for protein and lipid metabolism. Moreover, Akt promotes the phosphorylation of several targets, among which are: (1) GSK3β (on Ser9, inhibitory site), which has a role in energy production; and (2) mTOR (on Ser2448, activating site), which regulates protein synthesis and autophagy. Within this axis, BVR-A works as scaffold protein facilitating the PDK1-mediated activation of Akt and the Akt-mediated inhibition of GSK3β. Arrows represent stimulation; lines represent inhibition. See text for more details.
