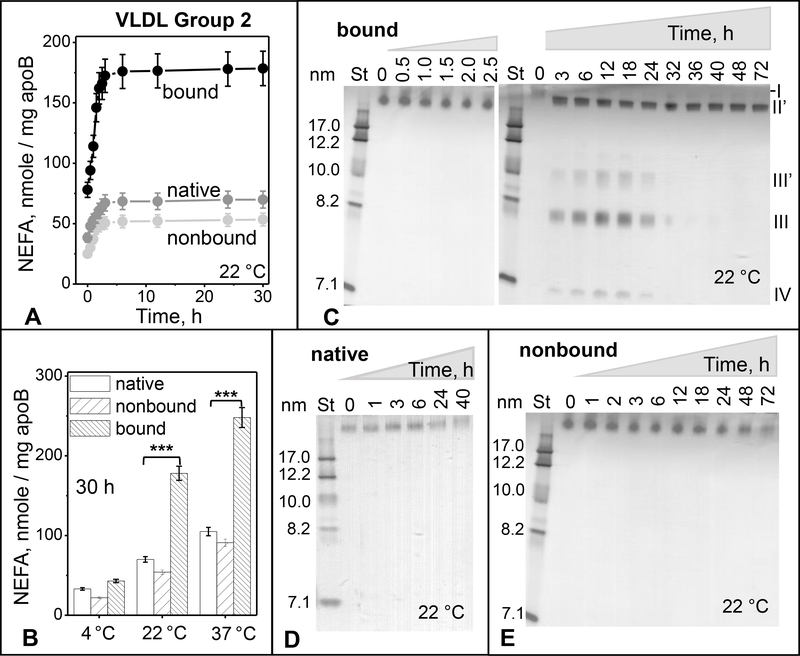
Figure 3.
Time and temperature dependence of spontaneous lipolysis and remodeling of group 2 VLDL.
(A) Time course of total lipolysis. Immediately after separation by heparin affinity chromatography, bound and non-bound VLDL fractions along with native VLDL (0.5 mg/ml total protein) were incubated in buffer A at 22 °C for up to 30 h (see part 2 Methods for details). NEFA levels were measured at indicated time points. The results are shown as the mean ±SEM of three independent measurements.
(B) Temperature dependence of total lipolysis. VLDL (0.5 mg/ml protein) in buffer A was incubated for 30 h at 4 °C, 22°C or 37 °C, whereupon NEFA were quantified as described in part 2 Methods. Statistically significant differences between bound and native VLDL are indicated (***, p≤0.05).
(C-E) Time course of particle remodeling at 22 °C for bound (C), native (D), and non-bound VLDL (E). VLDL (0.5 mg/ml protein) in buffer A was incubated for 42–72 h, sample aliquots were taken at indicated time points, cooled on ice, and immediately analyzed by non-denaturing PAGE. St – molecular size standards. Band positions corresponding to the size of intact VLDL (I), IDL/LDL (II’), HDL (III’ and III), and lipid-poor protein (IV) are indicated in panel C.