Abstract
Soil contamination with trace metal(loid) elements (TME) is a global concern. This has focused interest on TME-tolerant plants, some of which can hyperaccumulate extraordinary amounts of TME into above-ground tissues, for potential treatment of these soils. However, intra-species variability in TME hyperaccumulation is not yet sufficiently understood to fully harness this potential. Particularly, little is known about the rhizosphere microbial communities associated with hyperaccumulating plants and whether or not they facilitate TME uptake. The aim of this study is to characterize the diversity and structure of Arabidopsis halleri rhizosphere-influenced and background (i.e., non-Arabidopsis) soil microbial communities in four plant populations with contrasting Zn and Cd hyperaccumulation traits, two each from contaminated and uncontaminated sites. Microbial community properties were assessed along with geographic location, climate, abiotic soil properties, and plant parameters to explain variation in Zn and Cd hyperaccumulation. Site type (TME-contaminated vs. uncontaminated) and location explained 44% of bacterial/archaeal and 28% of fungal community variability. A linear discriminant effect size (LEfSe) analysis identified a greater number of taxa defining rhizosphere microbial communities than associated background soils. Further, in TME-contaminated soils, the number of rhizosphere-defining taxa was 6-fold greater than in the background soils. In contrast, the corresponding ratio for uncontaminated sites, was 3 and 1.6 for bacteria/archaea and fungi, respectively. The variables analyzed explained 71% and 76% of the variance in Zn and Cd hyperaccumulation, respectively; however, each hyperaccumulation pattern was associated with different variables. A. halleri rhizosphere fungal richness and diversity associated most strongly with Zn hyperaccumulation, whereas soil Cd and Zn bioavailability had the strongest associations with Cd hyperaccumulation. Our results indicate strong associations between A. halleri TME hyperaccumulation and rhizosphere microbial community properties, a finding that needs to be further explored to optimize phytoremediation technology that is based on hyperaccumulation.
Keywords: metal accumulation, microbial diversity, plant growth promoting bacteria, pseudometallophyte, soil metal contamination, trace metal(loid) element
Graphical Abstract

1. INTRODUCTION
Anthropogenic activities such as mining and smelting significantly contribute to the local accumulation of harmful trace metal(loid) elements (TME) levels in the environment. There is a global legacy of contaminated mine tailings that are eroded and transported by wind and water into nearby ecosystems. Smelting additionally results in TME distribution by releasing metal-rich particles into the atmosphere, which are then deposited downwind into local habitats (Balabane et al., 1999). The sustainable management of contaminants associated with mining and smelting of metal ores is regarded as a worldwide challenge that the mining industry faces (Virgone et al., 2018).
Establishing a lasting vegetation cover on contaminated sites is considered the best and most permanent way to minimize wind and water erosion and to stabilize mining impacted soils in situ (Mendez & Maier, 2008; Ali et al., 2013). Problematically, seed germination and plant growth are often inhibited under TME-rich conditions. Only a limited number of vascular plant species have evolved TME tolerance and are able to survive and reproduce in such environments (Baker, 1987). The majority of TME tolerant plants are labeled “excluders”, because their tolerance is based on minimizing metal(loid) uptake by the roots and limiting transport to shoots. Yet, certain species and populations are able to accumulate TME into above-ground tissues. Among these accumulators, a rare group called “hyperaccumulators” allocates extraordinarily large amounts of a given TME to their foliage, without showing any toxicity symptoms (Baker, 1981; Reeves & Baker, 2000). Importantly, the capacity for metal hyperaccumulation can differ among genotypes of the same plant species (Babst-Kostecka et al., 2018).
Hyperaccumulators provide an opportunity to study plant adaptation to extreme environments and they also have potential to be applied in the development of phytoremediation technologies, especially where metal removal or recovery is desired. There is increasing evidence that TME hyperaccumulation depends on a complex set of interactions among soil properties including TME bioavailability, expression of detoxification genes and metal transporters, as well as the plant-associated microbiome (Thijs et al., 2017; Asad et al., 2019; Trivedi et al., 2020). Importantly, it is known that plants enrich the rhizosphere with organic root exudates that in turn selectively attract and stimulate the growth of certain microbial taxa (Honeker et al., 2019b; Trivedi et al., 2020). Accordingly, different plant species may have a different rhizosphere microbiome when grown in the same soil, but also plants of the same species may harbor similar microbial communities in different soils (Miethling et al., 2000). Individual plant genotypes further differentiate their rhizosphere communities to best support the physiology, fitness, and improvement of plant responses to environmental stress (Bressan et al., 2009; Micallef et al., 2009; Yeoh et al., 2017; Trivedi et al., 2020). Although several studies have reported on these plant-microbial interactions in hyperaccumulating plants, particularly little is known about factors that lead to TME hyperaccumulation in non-metalliferous sites, where the environmental impact of this phenomenon has remained largely neglected (Lodewyckx et al., 2002; Li et al., 2007; Lopez et al., 2017). The rhizosphere-associated microorganisms, including bacteria, archaea, and fungi, are thus very important for their host and can facilitate plant performance and ecosystem functions in many ways (Mendes et al., 2013). In addition, there is evidence that they can enhance the TME-accumulation capacity of plants, thereby increasing TME content in shoot tissues (Farinati et al., 2009; Farinati et al., 2011; Muehe et al., 2015). Therefore, gaining deeper insight in these interactions across diverse soil types (metalliferous and non-metalliferous) is an important step towards further discerning the hyperaccumulation processes (Rosatto et al., 2019).
Arabidopsis halleri (L.) O’Kane and Al-Shehbaz is considered a model species for its ability to tolerate and hyperaccumulate excessive quantities of zinc (Zn) and cadmium (Cd; Roosens et al., 2008). It can tolerate high TME concentrations in contaminated soils (metallicolous [M] plant populations), but also thrives under natural conditions on uncontaminated soils (non-metallicolous [NM] plant populations). A broad quantitative variation in tolerance and hyperaccumulation of Zn and Cd has been observed in A. halleri across this range of environments (Bert et al., 2000; Bert et al., 2002; Talke et al., 2006; Meyer et al., 2010; Stein et al., 2017; Babst-Kostecka et al., 2018). For Zn and Cd, hyperaccumulation has been defined as over 3000 and 100 mg kg−1, respectively (van der Ent et al. 2013). Previous work has shown that while Zn and Cd tolerance levels are usually higher in M populations, NM populations on uncontaminated soils accumulate metals more efficiently than M populations on TME-contaminated soils. Thus, extremely high concentrations are found in A. halleri shoots, even when the element is present only at low concentration in the soil, indicating active TME foraging (Dietrich et al., 2019). We hypothesize that the associated soil microbiome in this case helps increase both plant TME accumulation capacity and plant fitness in A. halleri.
In this study, we characterized A. halleri rhizosphere-influenced and background soil microbiomes in locations from TME-contaminated and uncontaminated field sites. We selected four plant accessions from Southern Poland, for which different TME hyperaccumulation capacities have recently been reported (Babst-Kostecka et al., 2018; Dietrich et al., 2021). These data were combined with multiple physicochemical soil variables and host-plant traits to test for plant-soil-microbe associations. Based on this setup, following questions are addressed: (1) Are differences in A. halleri Zn and Cd hyperaccumulation capacities associated with variation in rhizosphere-influenced microbial community structure, diversity, and richness? (2) Are there specific bacterial, archaeal, or fungal rhizosphere phylotypes that associate with different hyperaccumulation patterns, plant accessions, and/or TME soil contamination? (3) Do the microbial phylotypes differentiating A. halleri rhizosphere-influenced and background communities differ between soils from TME-contaminated vs. uncontaminated sites?
2. MATERIAL AND METHODS
2.1. Sampling and climatic data
Four sites with naturally-occurring A. halleri were sampled in Southern Poland in late May 2018 (Table 1 and Fig. S1). Two were TME-contaminated sites, defined here as metalliferous (M), at low altitude in the Olkusz region (M_PL22 and M_PL27). Two were uncontaminated, defined here as non-metalliferous (NM); a sub-alpine location at the northern foothills of the Tatra Mts (NM_PL35) and a low altitude location in Niepołomice Forest (NM_PL14). The M sites differed in their history of industrial activity and source of TME contamination. Site M_PL22 was in the vicinity of the Zn smelter of the Bolesław Mine and Metallurgical Plant near Olkusz and site M_PL27 was near an open-pit mine that was closed in 1912 in Galman. Three replicates of both A. halleri rhizosphere-influenced and surrounding background soil were collected at each M and NM site. Rhizosphere-influenced (hereafter referred to as rhizosphere) samples were collected by first cutting away the aerial parts of the plant, followed by excavating soil at depth 0 to 15 cm to expose the plant roots. Roots were excised using sterile instruments, placed into a sterile plastic bag, and shaken to separate and collect ~ 50 g of soil that adhered to the roots for DNA analysis (Solís-Dominguez et al., 2012). An additional ~ 50 g of soil was sampled from the top 15 cm of the soil profile next to the A. halleri roots for geochemical analysis and placed into a plastic bag. To collect background samples at each site, three 5 × 5 m quadrats that were devoid of A. halleri vegetation were marked. For each quadrat, five ~ 50 g samples were taken at 10–15 cm depth devoid of visible plant roots (one from each corner and the center) and composited (Reimann et al., 2008). Subsamples for DNA analysis were taken as described by Kushwaha et al. (2021). All samples for DNA analysis were immediately placed on ice, transported back to the lab, and stored at −80°C until DNA extraction. Samples for geochemical analysis were stored at room temperature. After collecting the rhizosphere samples, roots and corresponding A. halleri shoots were sampled for elemental composition analysis. Shoots and roots were washed, dried at 80°C, and stored at room temperature. Note, that to avoid clonal replicates of A. halleri plants, the distance between samples was at least 5m.
Table 1.
Geographic location, pH, bioavailable and total Zn and Cd concentrations in rhizosphere soil samples (mean ± SD, n=3) at the four study sites.
| Site | Location | Latitude [°N] | Longitude [°E] | Elevation (m) | pH | ZnCDGT1 (μg L−1) | CdCDGT1 (μg L−1) | Total Zn (mg kg−1) | Total Cd (mg kg−1) |
|---|---|---|---|---|---|---|---|---|---|
| NM_PL14 | Niepołomice | 50.108833 | 20.367467 | 188 | 5.8 ± 0.6 | 12 ± 5 | 0.08 ± 0.01 | 127 ± 35 | 0.31 ± 0.11 |
| NM_PL35 | Kościelisko | 49.287056 | 19.879417 | 927 | 4.5 ± 0.3 | 35 ± 33 | 0.17 ± 0.06 | 61 ± 28 | 0.2 ± 0.1 |
| M_PL22 | Bukowno | 50.282800 | 19.478717 | 339 | 7.3 ± 0.1 | 1052 ± 260 | 13 ± 5 | 6068 ± 4916 | 45.2 ± 37.2 |
| M_PL27 | Galman | 50.198367 | 19.538817 | 471 | 6.1 ± 0.2 | 614 ± 335 | 8.6 ± 4.5 | 9401 ± 3160 | 102.4 ± 23.7 |
ZnCDGT and CdCDGT = bioavailable Zn and Cd
NM = non-metalliferous sites; M = metalliferous sites.
Monthly precipitation and air temperature data for the period 1997–2016 were obtained from meteorological databases at the Polish Institute of Meteorology and Water Management – National Research Institute (IMGW-NRI). Specifically, data were used from the meteorological station nearest to each of the four study sites (all within 15 km): Igołomia, Kościelisko-Kiry, Maczki, and Olewin meteorological stations for NM_PL14, NM_PL35, M_PL22, and M_PL27, respectively. Three bioclimatic variables were generated from the monthly temperature and precipitation data, averaged from 1997–2016: mean annual temperature (°C), annual precipitation (mm), and length of growing season (days).
2.2. Chemical analyses of plant material and geochemical analyses of soil
For elemental composition analysis, 0.5 g of dry-ground plant material was mixed with 10 ml of HNO3 (69–70%) and HClO4 (70–72%) 4:1 v/v, left for 24h, and mineralized at 290°C (FOSS Tecator Digestor Auto). Total Zn, Cd, Pb, Cu, Fe K, Na, Mg, and Ca concentration was determined using flame or graphic furnace atomic absorption spectrometry (AAS; Varian AA280FS, AA280Z, Agilent Technologies, Santa Clara, USA). Phosphorus concentration was determined by the vanadium-molybdenum method of Barton (1948) using 0.5 g of dry-ground material dissolved in HClO4, mineralized at 290°C and mixed with vanadium-molybdenum mixture and water. Absorbance at 490 nm were read using a Hach-Lange DR3800 spectrophotometer. The Zn, Cd and Pb translocation factors (TF) were determined as the ratio between metal concentration in shoots and roots.
Only rhizosphere soil samples were analyzed for pH, electrical conductivity (EC), total nitrogen (TN), total organic carbon (TOC), total carbon (TC), total inorganic carbon (TIC), NO2−-N, NO3−-N, NH4+-N, available P (P-Olsen), total Zn, Cd, Pb, Cu, Fe, Mg, Ca, K and Na; bioavailable Cd, Pb, Zn, and soil texture. Samples were sieved at 2 mm and dried. Soil pH (ISO 10390) and EC (PN-ISO 11265) were measured in 1:5 (w:v) water suspensions with a Hach HQ40D meter. Total N was determined using the Kjeldahl method; soil was digested in H2SO4 with Kjeltabs (K2SO4 + CuSO4•5H2O; Foss Tecator Digestor Auto) followed by distillation on a Foss Tecator Kjeltec 2300 Analyzer Unit. Total organic C, TC and TIC was determined with a dry combustion analyzer Leco RC-612 (ISO 10694). To analyze NO2−-N, NO3−-N, and NH4+-N concentrations, soil samples were shaken in water for 1 h (1:10, w:v), filtered through cellulose acetate membrane syringe filters (Huang & Schoenau, 1998) and anions in the extracts were determined with an ion chromatograph Dionex ICS-1100, while NH4+-N was determined with Dionex DX-100. P-Olsen was measured with an ion chromatograph (Dionex ICS-1100, Thermo Fisher Scientific) following soil extraction with 0.5 M NaHCO3. In order to determine total Zn, Cd, Pb, Cu, Fe, Mg, Ca, K and Na concentrations, ground samples were digested in hot concentrated HClO4 (FOSS Tecator Digestor Auto) at 288 – 292°C. Extracted elements were analyzed by flame or graphic furnace AAS as described above. Bioavailable fractions of Zn, Cd and Pb were assessed using Diffusive Gradients in Thin-films (DGT) method following the protocol provided by Dietrich et al. (2021). Soil texture was determined through a combination of sieving and sedimentation (ISO 11277).
2.3. Extraction of nucleic acids, amplicon sequencing and data processing
DNA was extracted from 0.5 g soil using the FastDNA Spin Kit for Soil™ (MP Biomedicals, Solon OH, USA) as modified by Kushwaha et al. (2021). Soil samples were thawed on ice prior to extraction. Extracts were purified using DNeasy PowerClean Pro Cleanup kit (Qiagen, Hilden, Germany) to remove inhibitors and the DNA was quantified using a Qubit 2.0 Fluorometer and double stranded DNA (dsDNA) high sensitivity assay kit (Invitrogen, Carlsbad, California, USA). All DNA extraction steps were performed with negative control samples (blanks) containing only reagents. Bacterial/archaeal 16S rRNA gene primers 515F/806R and fungal internal transcribed spacer (ITS) primers ITS1f-ITS2 were used for paired-end amplicon sequencing of DNA extracts as described in Walters et al. (2016). The purified amplicons from all the samples were pooled in equimolar concentrations and sequenced with a paired-end read length of 2 × 150-bp on the Illumina MiSeq platform. The DNA library preparation and sequencing runs were conducted by the Microbiome Core at the Steele Children’s Research Center, University of Arizona.
Raw reads were demultiplexed using the idemp tool (https://github.com/yhwu/idemp) and bioinformatics analysis was conducted using the DADA2 pipeline (Callahan et al., 2016). Demultiplexed reads were trimmed to the same length of 140 bases for the forward and reverse reads. The paired-end reads were merged using the default overlap of at least 12 bases and then grouped into amplicon sequence variants (ASVs). After removing poor quality and chimeric ASVs, a total of 1,336,123 and 3,353,095 sequence reads remained for the 16S rRNA and ITS genes respectively, with an average of 51,389 ± 23,065 (16S rRNA) and 128,965 ± 81,943 (ITS) reads per sample. Taxonomy identities were assigned to bacterial/archaeal and fungal ASVs using the SILVA (Quast et al., 2013) and UNITE ITS (Nilsson et al., 2018) databases, respectively. After removal of the contaminants from the ASV tables through comparisons of samples and the blanks, 11,665 and 6,166 ASVs were retained for analysis of bacterial/archaeal and fungal communities, respectively. Taxonomy tables were normalized using a cumulative-sum scaling approach prior to conducting statistical analysis (Paulson et al., 2013). The raw sequencing data for the 16S rRNA gene and ITS obtained in this study have been submitted to the NCBI BioProject number: PRJNA706064.
2.4. Statistical analyses
Microbial richness (number of observed ASVs), Shannon diversity index, and community dissimilarity were determined using the vegan package (Oksanen et al., 2008). Bray-Curtis distance was used to calculate community dissimilarity and ordination plots were visualized using non-metric multidimensional scaling (NMDS). The differences in richness metrics and Shannon diversity index across site type (M vs. NM), location (NM_PL14, NM_PL35, M_PL22, and M_PL27), and sample type (rhizosphere vs. background soil) were tested using Wilcoxon and Kruskal-Wallis tests. The differences in microbial community compositions between these groups were examined using nested permutational multivariate analysis of variance (PERMANOVA; Anderson, 2001). The factor location was nested within site type, and sample was nested within location. Location and sample were considered as random factors. Further, a linear discriminant effect size (LEfSe) analysis (Segata et al., 2011) was performed to identify indicator microbial taxa most likely to explain the differences between i) M and NM sites and ii) rhizosphere vs. background soils from both M and NM sites (see http://huttenhower.sph.harvard.edu/galaxy/root). A logarithmic cutoff value of linear discriminant analysis (LDA) > 2.0 was applied for LEfSe analysis. Additionally, a similarity of percentages (SIMPER) analysis was performed to determine which ASVs contributed the most to the average dissimilarity in microbial community structure between rhizosphere and background soil samples (iterations=1000). SIMPER was conducted separately for samples from M and NM sites. Only ASVs with permutation p-values < 0.05 are reported. The LEfSe method identifies statistically significant microbial indicators across each group and it weights the uniqueness of the taxon rather than its overall abundance, whereas SIMPER analysis reports the specific ASVs that contribute most to the average dissimilarity between groups and weights the abundance of individual taxa.
Non-parametric Kruskal-Wallis analysis was used to test for differences among the four sites with respect to abiotic and biotic soil properties, Zn and Cd shoot concentrations, and root-to-shoot translocation factors. Partial Least Square (PLS) regression was used to investigate the extent to which geography, climate, as well as biotic and abiotic soil and plant parameters explained variation in Zn and Cd hyperaccumulation. PLS is particularly well suited for situations where the number of predictors exceeds the number of observations (Carrascal et al., 2009), as is the case in our study. The PLS was implemented using function plsreg2 for multivariate cases from the plsdepot package in R (Sanchez & Sanchez, 2012). For each element, the analyses were run on two blocks of variables: a matrix of 95 predictors and a matrix of two responses (i.e., Zn shoot concentration and ZnTF for the Zn hyperaccumulation trait; and Cd shoot concentration and CdTF for the Cd hyperaccumulation trait). The predictors included: six geographic and climatic variables, nine elemental plant shoot concentrations, as well as 25 geochemical and 55 biological properties of corresponding rhizosphere samples. The biological properties included: i) microbial diversity metrics, e.g., richness, Shannon diversity index, and NMDS axes scores, ii) relative abundance of indicator microbial taxa likely to explain the differences between rhizosphere vs. background soil samples (rhizosphere taxa with LDA > 4.0 as per LEfSe analysis), and iii) relative abundance of microbial taxa that contributed to the average dissimilarity in microbial community structure (permutation p-values < 0.05 as per SIMPER) between rhizosphere and background soil samples from M and NM sites. A heatmap of the relative abundance of taxa identified as significantly associated with Zn and Cd hyperaccumulation was generated using ggplot2 in R.
It is noted that geographic and climatic data were represented by a single value per population and that each value was replicated for all samples from the same sampling site. In the PLS analyses, the optimal number of components was determined by leave-one-out cross validation. Cumulative R2 (%) values are provided according to the retained number of components. These values reflect the explanatory power of the components for all dependent variables (cumulative R2Y) and for all explanatory variables (cumulative R2X). Predictors that contributed most to the underlying variation in metal hyperaccumulation were identified based on their “Variable Importance in Projection” (VIP) scores. Accordingly, variables with VIP scores greater than 0.8 were considered critical in a given PLS regression model (Farrés et al., 2015). Effect direction and intensity of each variable are specified by the sign and the absolute value of the corresponding standardized and scaled regression coefficients. All statistical analyses were performed using R 3.6.0 (R Core Team, Vienna, Austria) and XLSTAT.
3. RESULTS
Geographic and climatic data for the sampling sites, elemental plant shoot concentrations, and geochemical properties of rhizosphere soil samples are provided in Supplementary Table S1.
3.1. General characterization of plant Zn and Cd hyperaccumulation
All populations, independent of their edaphic origin, accumulated significantly more Zn (Fig. 1A, 1B) and Cd (Fig. 1E, 1F) in shoot than in root tissues. For Zn, the mean Znshoot concentration was on average twice as high in M than in NM plants (Table S1). Individual values ranged from 2013 mg kg−1 (NM_PL35) to 13,254 mg kg−1 (M_PL27), with only two NM_PL35 samples not reaching the Zn hyperaccumulation threshold (3000 mg kg−1; van der Ent et al. 2013). Interestingly, Znshoot concentrations in NM_PL14 plants did not differ from M populations (Fig. 1A), despite ~80-fold lower total and bioavailable Zn soil concentrations at the NM_PL14 site (Fig. 1D; Table 1). Further, bioavailable Zn soil concentrations were significantly higher at M sites when compared to NM sites (Fig. 1D). For Cd, M plants had on average nine-fold higher Cdshoot concentrations compared to NM plants. While all M plants hyperaccumulated Cd, none of the NM plants met the hyperaccumulation threshold for this metal (100 mg kg−1; van der Ent et al. 2013). All NM and M plants had translocation factors (ZnTF and CdTF) that exceeded one (Fig. 1C, 1G). Overall, Zn and Cd translocation factors were higher in M compared to NM populations; however, the difference was only significant for Cd translocation in NM vs. M_PL27. Similar to Zn soil concentrations, bioavailable Cd soil concentrations were significantly higher at M sites when compared to NM sites; in addition, the NM_PL35 had significantly higher Cd soil concentrations than NM_PL14 site (Fig. 1H).
Figure 1.
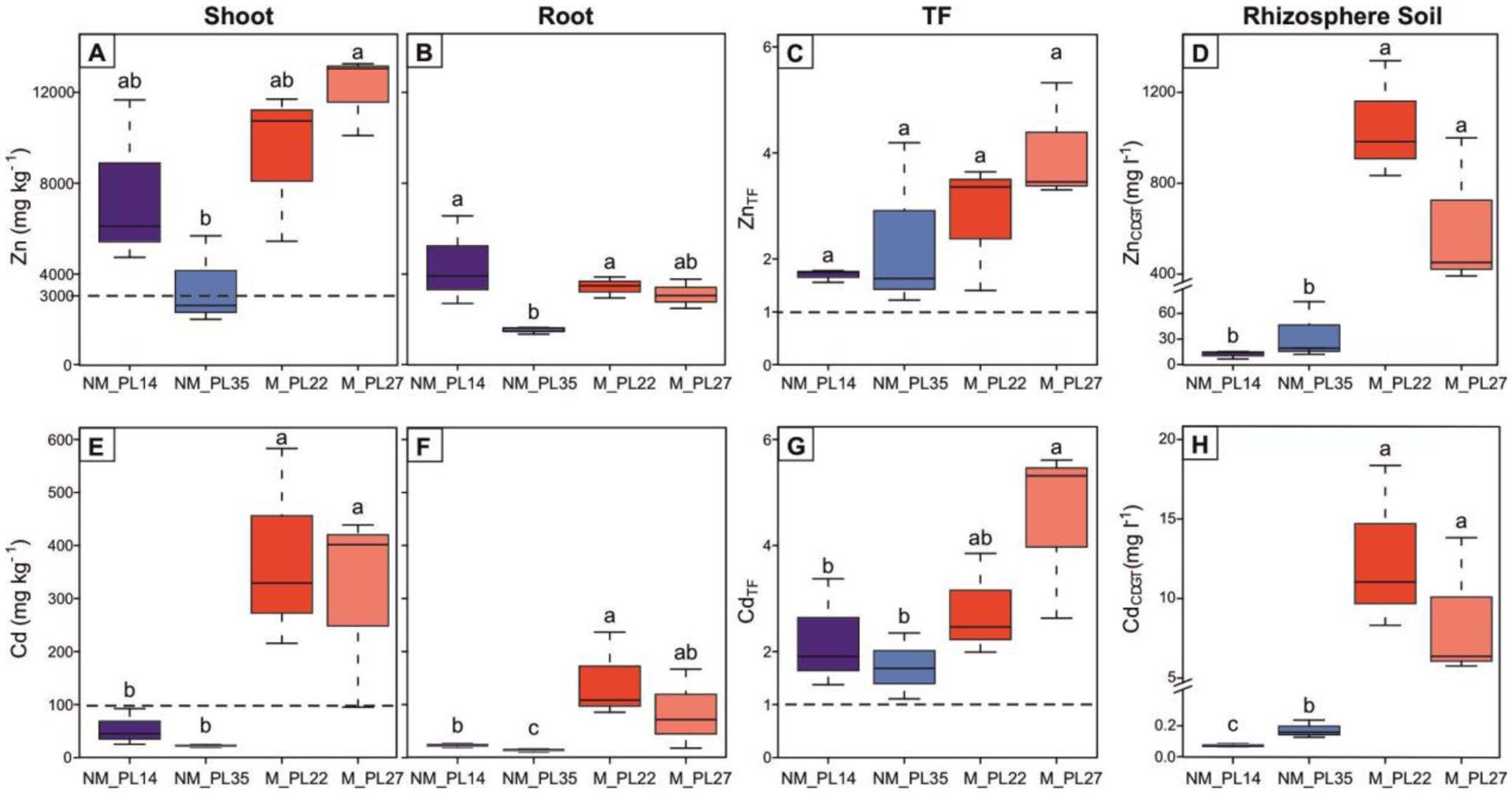
Uptake of Zn and Cd by A. halleri in the four study sites. Shoot and root concentrations of Zn (panels A and B, respectively) and Cd (panels E and F, respectively), root-to-shoot translocation factors (TF) for Zn (panel C) and Cd (panel G), and bioavailable (CDGT) fractions of Zn (panel D) and Cd (panel H) in rhizosphere soil are shown for each site. Each box represents the inter-quartile range of the data, with the median indicated by the horizontal line. The dotted lines in panels A and E indicate the thresholds for hyperaccumulation for Zn and Cd, respectively. The dotted lines in panels C and G indicate a TF = 1. NM = non-metalliferous and M = metalliferous soils. Different letters indicate statistically significant differences at p ≤ 0.05 (Kruskal-Wallis test).
3.2. Microbial community diversity and structure
When rhizosphere and background soil samples were analyzed together, neither bacterial/archaeal nor fungal richness were significantly different across the four study sites (Fig. 2A, Table S2). In contrast, community ordination analysis showed that site type (M vs. NM) and location (NM_PL14, NM_PL35, M_PL22, M_PL27) explained 26% and 18%, respectively, of the variation in bacterial/archaeal community composition (p = 0.001), as well as 18% and 10% of the variation in fungal community composition (p = 0.001) (Fig. 2B). Overall, in this analysis 44% and 28% of the community variability for bacteria/archaea and fungi, respectively, could be explained by the combination of site type and location. Wilcoxon test revealed no significant variation for the richness and Shannon diversity index for microbial communities between rhizosphere and background soil (Table S2).
Figure 2.
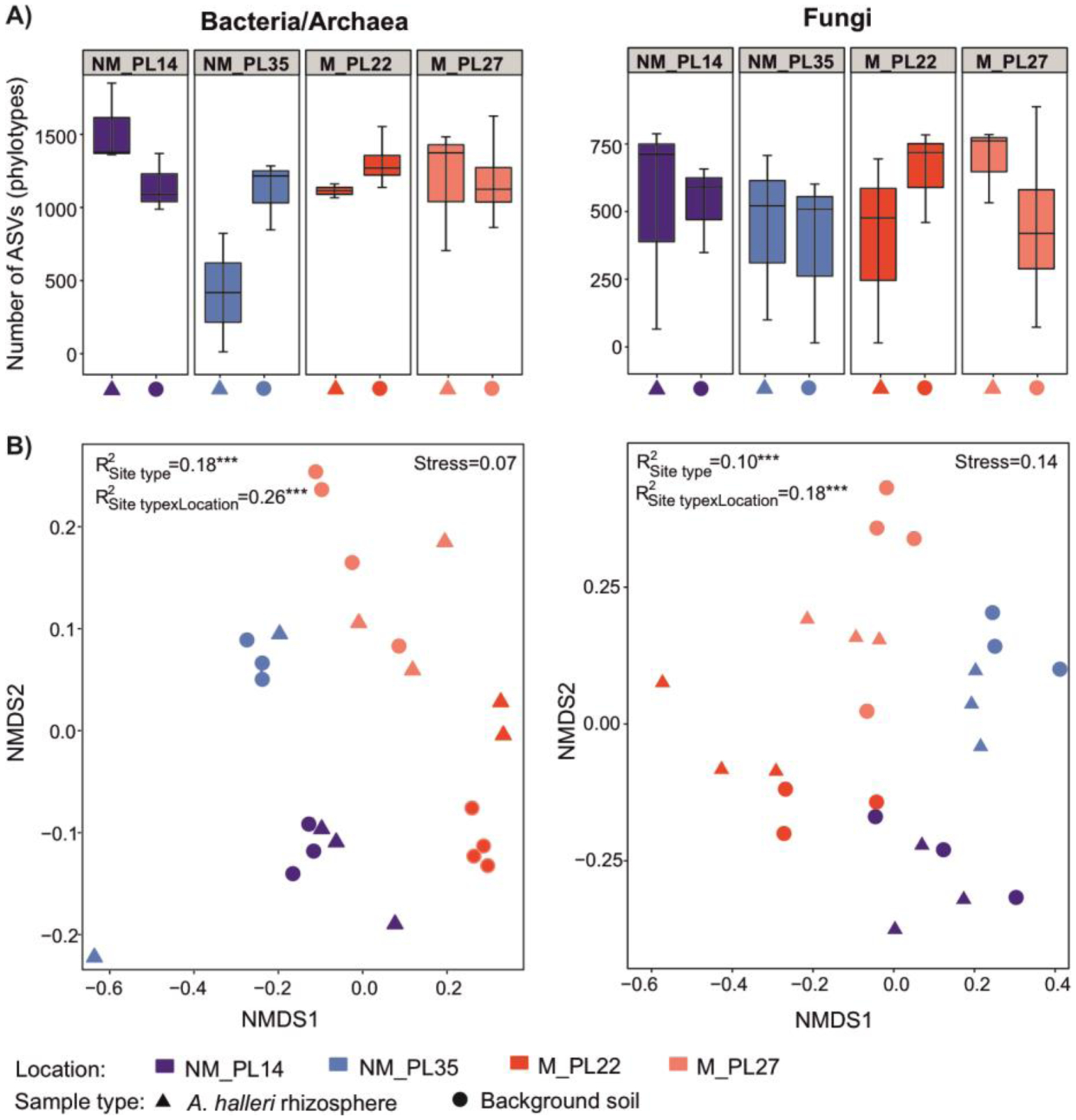
(A) Number of observed bacterial/archaeal and fungal amplicon sequence variants (ASVs) or phylotypes in the four study sites for rhizosphere and background soils. The boxes represent the inter-quartile range of the data, the median is indicated by the horizontal line. No comparisons between the means within the A panel were significant (Wilcoxon and Kruskal-Wallis tests). (B) Nonmetric multidimensional scaling (NMDS) ordination plots of microbial community structure in the four study sites. R2 represents the variation explained by site type (M vs. NM) and location (NM_PL14, NM_PL35, M_PL22, M_PL27) for the microbial community composition (p-value of 0.001 is represented as ***; PERMANOVA). NM = non-metalliferous and M = metalliferous soils.
3.3. Key microbial taxa differentiating rhizosphere and background soil samples
LEfSe and SIMPER analyses were conducted to identify defining taxa that differentiate the microbial communities of M and NM soils and their respective rhizosphere and background soil communities. LEfSe weights the uniqueness of a taxon within a community, whereas SIMPER weights the relative abundance. Results showed that M sites had four times as many unique bacterial/archaeal ASVs and almost twice the number of fungal ASVs than were found in NM sites (Table 2). As a result of this difference, the rhizosphere and background soil communities were analyzed separately for the M and NM sites. Overall, the analyses showed a higher number of microbial taxa defining the rhizosphere communities than the background soil communities (Table 2). Further, this difference was more pronounced in M sites for which the rhizosphere: background ratio of unique taxa was almost 6 for both bacteria/archaea and fungi. The corresponding ratio for NM sites, was 3 and 1.6 for bacteria/archaea and fungi, respectively (Table 2).
Table 2.
Number of taxa identified by linear discriminant effect size (LEfSe) analysis that explain the differences between contaminated and uncontaminated site types and between rhizosphere and background soil communities within a given site type.
| Compared Sample Groups | Number of taxa in Sample Group 1 vs. Sample Group 2; (ratio) | ||
|---|---|---|---|
| Sample Group 1 | Sample Group 2 | Bacteria/Archaea | Fungi |
| M (contaminated sites) | NM (uncontaminated sites) | 20 vs. 5; (4) | 32 vs. 19; (1.7) |
| Rhizosphere soil (M sites) | Background soil (M sites) | 40 vs. 7; (5.7) | 57 vs. 10; (5.7) |
| Rhizosphere soil (NM sites) | Background soil (NM sites) | 24 vs. 8; (3) | 11 vs. 7; (1.6) |
The threshold on the logarithmic LDA score for discriminative features was set to 4, to evaluate the microbial taxa that were the most prominent in defining the differences between rhizosphere vs. background soils and M & NM sites. Accordingly, seven out of the 40 bacterial/archaeal taxa were identified as the top rhizosphere taxa at M sites, whereas rhizosphere taxa at NM sites had only one out of the 24 taxa with LDA > 4.0 (Figure S2; Table 2). The top bacterial/archaeal rhizosphere taxa at M sites included: Betaproteobacteriales, Burkholderiaceae, Gammaproteobacteria, Nitrosomonadaceae, Pseudonocardiaceae, Pseudonocardiales, and IS-44 (Fig. S2A; Table S3). In contrast, the top rhizosphere taxon at NM sites was only classified up to the bacterial kingdom level (Fig. S2B; Table S3). For M background soil community, the defining taxa belonged to the phyla Acidobacteria and Actinobacteria (LDA > 4.0). Interestingly, most of the defining NM background soil taxa belonged to Thaumarchaeota.
At M sites, LEfSe identified 57 fungal taxa in rhizosphere soil that distinguished the rhizosphere community from the background soil community (Fig. S3A; Table S3). The top six fungal taxa that associated with the M rhizosphere community were Ascomycota, including four taxa that belonged to Dothiodemycetes order (LDA > 4.0; Fig. S3A). Although the number of fungal taxa in the M rhizosphere soil community was higher, the number of bacterial/archaeal and fungal taxa with LDA > 4.0 was the same. Ten fungal taxa were identified by LEFSe as related to the background soil at M sites. These fungal taxa included the phyla: Ascomycota, Basidiomycota, Mortierellomycota, and Glomeromycota (Fig S3A). In contrast, only 11 fungal taxa were identified in the NM rhizosphere community and Cantharellales was the only one fungal taxon with LDA > 4.0 (Fig. S3B).
As with LEfSe, SIMPER analysis identified more taxa for M soils that contributed to the dissimilarity between rhizosphere and background soils than for the NM soils (Table S4). Twenty-three and 12 bacterial taxa differentiated the rhizosphere community from the background soil communities for M and NM sites, respectively (p < 0.05; Table S4). The genus Pseudomonas was the most significant taxa defining M rhizosphere samples, whereas the order Gaiellales was the most significant in NM samples. Regarding fungal communities, three taxa explained the differences between rhizosphere and background soil communities from M sites and two taxa from NM sites (p < 0.05; Table S4). The fungal species Russula depallens emerged as the most significant for M sites and its contribution towards the dissimilarity between rhizosphere and background soil communities was the highest among all taxa (1.4%). In NM soils, the fungal taxon from the Helotiales order had the highest contribution to the dissimilarity between rhizosphere and background soils.
3.4. Abiotic and biotic variables positively related to enhanced Zn and Cd hyperaccumulation
Leave-one-out cross-validation indicated that the optimal number of partial least square (PLS) components for both regression analyses (i.e., performed on either Zn hyperaccumulation or Cd hyperaccumulation traits) was two. These two components together explained 71% and 76% of the variance in Zn and Cd hyperaccumulation, respectively (Fig. 3A; Fig. S4). Importantly, as our regression models were based on the same numbers of components, they are fully comparable.
Figure 3.

Biotic and abiotic drivers of Zn and Cd hyperaccumulation as determined by partial least square (PLS) regression analysis. (A) Correlations of the trait (black) and explanatory (colored) variables with the first two axes associated with the first two PLS components. The inner dashed circle denotes the correlation coefficient r = 0.75. The percentages of the variances in the matrix of responses and in the matrix of predictors explained by each variable are indicated on the respective axes. Note that variables with longer lines have greater loadings in the first or second component and are thus more influential in the model. Angles between the lines reflect the correlation between the variables; smaller angles indicate that variables are highly correlated. (B) Standardized regression coefficients (St. coeff.) for explanatory variables relevant for explanatory variables relevant for Zn and Cd hyperaccumulation. Note that only variables with Variable Importance in Projection > 0.8 are shown. TF, translocation factor; Temp, mean annual temperature; Precip, annual precipitation; CDGT, bioavailable fractions; EC, electrical conductivity; TN, total nitrogen; TC, total dissolved carbon; TOC, total organic carbon; TIC, total inorganic carbon. The letters in front of the microbial taxa names reflect the level of taxonomic hierarchy: c, class; o, order; f, family; g, genus.
For Zn hyperaccumulation, all abiotic and biotic variables together (cumulative R2Y) and both Zn hyperaccumulation variables together (cumulative R2X from Znshoot and ZnTF) explained 50% and 47% of variance for the first PLS component and 21% and 11% for the second component, respectively. For Cd hyperaccumulation, cumulative R2Y and cumulative R2X (from Cdshoot and CdTF) explained 58% and 52% of variance for the first PLS component and 18% and 16% for the second component, respectively. To evaluate the importance of each predictor variable in our models, we used the VIP scores from the PLS output. Accordingly, 45 and 47 out of 95 variables were identified as relevant in the Zn and Cd hyperaccumulation PLS regression models, respectively (Table S5). The absolute values of the corresponding beta regression coefficients express the relative strength of these variables in explaining the variance in Zn and Cd hyperaccumulation (Fig. 3B).
A striking result from the PLS analysis is that the three out of the four strongest variables that impacted Zn hyperaccumulation (Znshoot and ZnTF) were fungi-related including: fungal richness, diversity, and the taxa Humicola (Fig. 3B). In contrast, none of these fungi-related variables were important for Cd hyperaccumulation. The only important fungi-related variable for Cd was related to CdTF and in this case, the taxa Dothideomycetes was the strongest impacting variable. Interestingly, Dothideomycetes was also a strong variable (10th ranked) for ZnTF (Fig. 3B). Other important variables correlated with Zn uptake included plant shoot concentration of Mg and several abiotic variables including NH4+-N, TOC, TC, TN, and Cu. Bacteria were of less importance as variables that impacted high levels of Zn uptake.
The results for Cd are in stark contrast to Zn. The strongest variables related to Cd hyperaccumulation were abiotic soil variables and bacteria. Abiotic variables of importance to enhanced Cdshoot concentrations included Cd and Zn bioavailability. Abiotic variables important for the CdTF included: Cu content, NH4+-N, TOC, and TC. Bacteria related to high levels of Cd uptake included Kineosporia and Lysinmonas for Cdshoot and Pseudomonas, Devosia, Flavobacterium, and Crossiella for CdTF. Though abiotic factors and bacteria were most important for Cd hyperaccumulation, the exception was that for CdTF the fungus Dothideomycetes was the most important factor.
3.5. Abiotic and biotic variables negatively related to Zn and Cd hyperaccumulation
Six out of the seven strongest variables that negatively associated with Zn hyperaccumulation (Znshoot and ZnTF) were the same including: NO3—N, latitude, bacterial taxa Nitrospira, Phenylobacterium, Subgroup2; and fungal taxa Helotiales Incertae sedis (Fig. 3B). Interestingly, bioavailable concentrations of Cd, Pb, and Zn along with pH negatively related to translocation of both Zn and Cd. Seven bacteria taxa negatively impacted CdTF, whereas Cd hyperaccumulation was related to fungal NMDS axis score and Dothideomycetes. Notably, different nitrogen forms influenced Zn and Cd hyperaccumulation with NO3−-N and NH4+-N relating to Zn and Cd uptake, respectively.
3.6. Key microbial taxa that associated with Zn and Cd hyperaccumulation
PLS analysis identified 29 microbial taxa (24 bacteria and 5 fungi) in rhizosphere as significant explanatory variables of Zn and Cd hyperaccumulation in A. halleri shoots (Fig. 3). Notably, some taxa were associated uniquely with Zn (7) or Cd (11) hyperaccumulation, whereas many were associated with both (11). The vast majority of these 29 taxa were present in the A. halleri rhizosphere at M sites, with 27 and 24 taxa linked to the M_PL27 and M_PL22 sites, respectively (Fig. 4). Only 13 of the taxa were found in the NM_PL35 site, but 21 taxa corresponded to the NM_PL14 site. Interestingly, there were 10 bacterial taxa that were present at both M sites and one NM site NM_PL14, but not at NM_PL35. The relative abundance of these taxa in NM_PL14 was either comparable or lower than in M sites. The bacterial taxa belonged to five phyla, including Acidobacteria, Actinobacteria, Bacteroidetes, Nitrospirae, and Proteobacteria and the five fungal taxa were from the Ascomycota phylum (Table S3). There were six bacterial genera (Actinomycetospora, Crossiella, Marmoricola, Bradyrhizobium, Rhodoplanes, and Pseudomonas) that had relative abundance > 0 in the M sites (M_PL22 and M_PL27) but were absent at NM sites (NM_PL14 and NM_PL35) (Fig. 4). In contrast, two bacterial genera, Nitrospira and Phelybacterium, were present exclusively at NM sites.
Figure 4.

Heatmap showing the relative abundance of microbial taxa in A. halleri rhizosphere from contaminated (M_PL22, M_PL27) and uncontaminated (NM_PL14, NM_PL35) locations. The 29 taxa identified as significant drivers of Zn and Cd hyperaccumulation (as determined by partial least square regressions) were considered for the relative abundance comparison. The letters in front of the microbial taxa names reflect the level of taxonomic hierarchy: c, class; o, order; f, family; g, genus. The taxa are arranged alphabetically in the order of their phylum.
4. DISCUSSION
4.1. Arabidopsis halleri metal hyperaccumulation patterns in M and NM sites
As shown recently, the four A. halleri populations (two each from M and NM sites) in this study are both genetically similar and in close geographic proximity (Babst-Kostecka et al., 2018). Here, we investigated the differences in Zn and Cd hyperaccumulation among these populations. All plants reached exceptionally high concentrations of both Zn and Cd in the shoots at M sites, whereas only Zn was hyperaccumulated at NM locations. These results are congruent with the findings of previous studies on A. halleri and underline the constitutive nature of Zn hyperaccumulation and the population-specific character of Cd hyperaccumulation in this species (Bert et al., 2000; Stein et al., 2017; Corso et al., 2018).
The evolutionary dynamics of Zn hyperaccumulation by A. halleri are particularly interesting, as illustrated by a recent study that associated an increase in Zn hyperaccumulation in lowland NM population in Niepołomice Forest with the colonization of this location by plants from a former M population (Babst-Kostecka et al., 2018). Similarly, in this study the NM_PL14 population (Niepołomice Forest) accumulated remarkably high Zn concentrations, matching levels found at M locations even though the soil Zn concentration at the NM_PL14 site was ~80-fold lower compared to M sites. Interestingly, the Niepołomice Forest accessions have reduced neutral genetic variation compared to other NM and M populations from the same geographic region (Babst-Kostecka et al., 2018), which could negatively impact population fitness (Markert et al., 2010). It is thus surprising that these genetically less diverse A. halleri plants showed no visible toxicity symptoms even after extremely high Zn accumulation and reached the highest aboveground biomass of all investigated populations from Southern Poland in an earlier study (Dietrich et al., 2019). Taken together, these results suggest that soil TME concentration is not the main driver for A. halleri Zn hyperaccumulation and led us to consider other possible biotic and abiotic factors that could influence TME uptake. These are discussed in the following sections.
4.2. Microbial community dynamics in M and NM soils
Elevated concentrations of TME are known to impact the soil microbial community. In this study, both M and NM sites were characterized by similar levels of microbial richness and diversity, but notable differences in microbial community composition were observed. Similarly, long-term exposure to historic metal-contaminated mine tailings has previously been reported to have weak or no impact on microbial alpha diversity in soils (Gans et al., 2005; Bamborough & Cummings, 2009). This was attributed to the local adaptation of microbial communities and the replacement of metal sensitive groups by more tolerant ones (Berg et al., 2012; Azarbad et al., 2015). In terms of microbial community structure, differences in the composition of both bacterial/archaeal and fungal communities in M and NM sites were observed both in the present and previous studies (Tipayno et al., 2018; Xu et al., 2019). As suggested above, these differences may be due to the replacement of metal-sensitive with metal-tolerant microbial populations.
Not only did metal contamination (M vs. NM sites) affect the structure of bacterial/archaeal and fungal communities differently in this study but the contribution of site type in shaping the community composition was two-fold greater for bacteria/archaea than for fungi. A similar pattern was previously reported by Khan et al. (2010) and may be associated with differences in bacterial and fungal activities in M soils (Rajapaksha et al., 2004). Further, phospholipid fatty acid analysis – commonly used to quantify soil microbial responses to environmental stress – has shown positive correlation between soil available Cd and fungal indicators but for bacterial indicators the correlation is a negative one (Shentu et al., 2014).
Of particular interest were the observed differences between A. halleri rhizosphere and background soil microbial communities from M and NM sites. In particular, the number of microbial taxa defining the rhizosphere was greater than for background soils. This difference was more pronounced in M than in NM sites. One possible explanation for these findings is that A. halleri recruits a more unique rhizosphere community from the background soil in M vs. NM sites. This is supported by Honeker et al. (2019), who reported a greater number of taxa enriched in Atriplex lentiformis rhizosphere in acidic pyritic mine tailings (24 rhizosphere taxa vs. 0 bulk taxa) compared to higher pH (5.2–7.8) substrates (15 rhizosphere taxa vs. 3 bulk taxa). Similar observations were derived from comparisons between A. lentiformis rhizosphere and bulk taxa in compost-amended and unamended pyritic tailings (Valentín-Vargas et al., 2018). Taken together, these findings indicate that plants growing under M conditions recruit a greater number of novel rhizosphere taxa than their counterparts in NM soils.
Another possible explanation for a higher number of unique defining rhizosphere taxa in M sites may be the presence of specific metal tolerant or plant growth promoting bacteria (PGPB) that facilitate survival and help alleviate plant metal stress (Ma et al., 2015). Overall, PGPB can enhance plant growth by regulating plant stress responses through the production of siderophores, phytohormones, and enzymes (Penrose & Glick, 2001; Press et al., 2001; Patten & Glick, 2002). The present study identified several bacterial groups with the above-mentioned PGPB properties within the taxa associated with the A. halleri rhizosphere from M sites, including Burkholderiaceae and Sphingomonadaceae. For instance, the Pandoraea genus of Burkholderiaceae is known to produce the enzyme 1-aminocyclopropane-1-carboxylate (ACC) deaminase that alleviates stress by lowering ethylene concentrations in plants exposed to biotic and abiotic stress (Anandham et al., 2008). Genera of Sphingomonadaceae can produce phytohormones like gibberellins, abscisic acid, indole-3-acetic acid, and salicylic acid to promote plant growth (Yang et al., 2014). Taken together, the production of phytohormones and enzymes by rhizospheric PGPB are important adaptive strategies that are likely to facilitate the success of A. halleri survival and growth in M soils.
The fungal taxa identified as most likely to explain differences between A. halleri rhizosphere and surrounding background soils were almost 6-fold higher in M than in NM sites (57 vs. 11 fungal taxa). Out of 57 unique rhizosphere fungal taxa associated with M sites, the existing literature characterizes very few with defined functional roles to support plants. Indeed, previous efforts that have characterized fungi in M soils and described fungal metal-tolerance mechanisms did not report on many of the specific taxa identified in our study (Miransari, 2010; Zarei et al., 2010; Miransari, 2011; Luo et al., 2014; Thijs et al., 2017). Exceptions include selected species of the genera Hormonema and Phialocephala which were reported to produce the auxin phytohormone indole-3-acetic acid and siderophores, respectively (Bartholdy et al., 2001; Soto et al., 2019). Given the number of novel fungal taxa that we found in the A. halleri rhizosphere from M sites, but not NM sites, the importance of fungi in Zn uptake (Fig. 3), and the unknown functional role of most of these taxa, further research is needed to unravel their potential roles in the rhizosphere of metal-hyperaccumulating plants.
4.3. Zn and Cd hyperaccumulation are shaped by different biotic and abiotic parameters
We observed a large variability in the capacity for hyperaccumulation of Zn and Cd in M and NM populations of A. halleri from Southern Poland. While such behavior is well documented in the literature, the factors that drive this variation are not yet well-understood (Honjo & Kudoh, 2019). Recent studies have linked the concentration of Zn and Cd accumulated in plant shoots with soil element composition showing that soil metal concentrations explain only a small part of the variation in both hyperaccumulation traits (Stein et al., 2017; Frérot et al., 2018). To further investigate factors important for Zn and Cd hyperaccumulation this study measured 95 abiotic and biotic parameters. Results show that Zn hyperaccumulation was predominantly governed by biotic variables and similar factors were associated with both Zn shoot accumulation and the Zn translocation factor. Specifically, the strongest positive association of Zn hyperaccumulation was with fungal richness and diversity. This was followed by abiotic factors including ammonium N, total organic C, and total N. The strongest negative associations of Zn hyperaccumulation were with nitrate N and some bacterial taxa. In contrast with Zn, Cd hyperaccumulation was primarily explained by abiotic factors and different factors were more strongly associated with either Cd shoot accumulation or the Cd translocation factor. The strongest positive association of Cd in shoot tissues was with the bioavailability of Cd, Zn, and Pb. Similarly, concentrations of Cd and Pb in soil were previously reported to be strong drivers of the evolution of Cd hyperaccumulation in A. halleri (Frérot et al., 2018). Also strongly positively associated with Cd in shoot tissues were several bacteria/archaea taxa which are discussed further below. The variables with the strongest positive association with CdTF were a single fungal taxon, and three abiotic variables including soil Cu concentration, ammonium N, and total organic C.
Recall that Cd was hyperaccumulated only at M sites. Several bacterial taxa that were abundant in the A. halleri rhizosphere at both M sites, but not at the NM sites, were strongly associated with Cd hyperaccumulation. These include: Actinomycetospora, Bradyrhizobium, Marmoricola, and Pseudomonas. Pseudomonas is a well-known PGPB and has previously been reported to reduce metal-induced stress in plants at M sites (Xiao et al., 2017; Honeker et al., 2019). Cadmium-resistant Pseudomonas sp. strains are capable of either leaching out Cd from Cd-complexed compounds by producing organic acids or biosorbing metals by releasing Cd-binding siderophores and peptides (Muehe et al., 2015). Some strains have also been shown to have a positive effect on the phytoextraction of Cd and Zn. Indeed, inoculation with Pseudomonas sp. strains increased Cd accumulation and stimulated the growth of roots and shoots in the Zn/Cd/Pb hyperaccumulator Brassica napus (Sheng & Xia, 2006; Dell’Amico et al., 2008; Dąbrowska et al., 2017), and increased growth and Cd and Zn content in Zn/Cd hyperaccumulator Sedum alfredii (Li et al., 2007). Also Bradyrhizobium, which is typically involved in atmospheric N fixation (Swanner and Templeton 2011), can produce siderophores and thus has the potential to increase metal solubility in the rhizosphere of metal hyperaccumulating plants (Asad et al., 2019).
A second grouping of bacteria with PGPB activities includes taxa which were associated with both Zn and Cd hyperaccumulation: Rhodoplanes, Crossiella, and Solirubrobacteraceae, the latter two belonging to Actinobacteria phylum. While some Rhodoplanes are known for their N-fixation potential (Zhu et al., 2018), they are also capable of producing plant growth hormones (indole-3-acetic acid and 5-aminolevulinic) (Sun et al., 2015) that can alleviate metal-induced toxicity and stress in plants. Actinobacteria can enhance plant growth and yield through the fixation of atmospheric N, the solubilization of minerals such as P, K, and Zn, as well as the production of siderophores and plant growth hormones.
Five bacterial/archaeal and fungal taxa were identified as negatively associated with hyperaccumulation of Zn and Cd in the shoots. These included taxa that were abundant solely in NM sites and belonged to the phylum Acidobacteria (order Subgroup2), genus Nitrospira and Phenylobacterium. Their restriction to NM soils suggests that these microorganisms might be sensitive to soil metal contamination. Indeed, previous studies showed that Acidobacteria, which are involved in various soil processes, are sensitive to M soils (Bell et al., 2015). In contrast, the well-known nitrite oxidizer Nitrospira has previously been shown to be adapted to M sites (Luo et al., 2018). Regarding Phenylobacterium, its functional relevance is currently unknown, however it was previously reported in the rhizosphere of A. halleri from an M soil (Muehe et al., 2015).
Finally, we note a group of 10 bacterial taxa that were present at both M sites (M_PL27 and M_PL22) and at the lowland NM site (NM_PL14), but not at NM_PL35. Given the remarkably high Zn hyperaccumulation levels in plants from NM_PL14, M_PL27 and M_PL22 locations, these microbial taxa may play a role in the mechanism of Zn hyperaccumulation. In particular, Devosia, which was widely abundant in our study at M and NM_PL14 sites, is a well-known N-fixing bacteria (Laranjo et al., 2014). Devosia has previously been associated with Zn hyperaccumulator Thlaspi caerulescens (Lodewyckx et al., 2002) and Ni hyperaccumulator Alyssum murale (Lopez et al., 2017). It has been shown to occur in both bulk and rhizosphere soils at higher pH (5.2–7.8), but was present exclusively in the rhizosphere of acidic soils (Honeker et al., 2019). Accordingly, Devosia seems to be recruited by plants-root systems that encounter toxic conditions and is likely to be a relevant member of the rhizobacterial community associated with hyperaccumulating plants. Other interesting taxa observed were Lysinimonas and Galbitalea from the Microbacteriaceae family. Strains of these metal-resistant bacteria were isolated from the rhizosphere of Salix caprea grown at a M site. They were shown to significantly increase the extractability of Zn and Cd from contaminated soil and cause an increase of Zn and Cd concentration in S. caprea shoots (Kuffner et al., 2008; De Maria et al., 2011). Finally, members of Chitinophagaceae were found in both M sites and in NM_PL14. Recent research identified Chitinophagaceae in the rhizosphere of Zn/Cd hyperaccumulator Sedum alfredii and showed a positive correlation with Cd and Pb concentration in plant shoots and roots (Cao et al., 2020). Overall, the presence and ecological function of A. halleri rhizosphere taxa in both M sites and NM_PL14 (but not NM_PL35) suggests that selected members of microbial communities may affect Zn and Cd mobilization in soils and result in exceptionally efficient Zn uptake by A. halleri plants at both M and NM locations. These findings highlight the importance of investigating the role of rhizosphere microbial diversity in metal mobilization at M as well as NM sites. Yet, the diversity and abundance of the rhizosphere microbial community as characterized by DNA amplicon sequencing may vary from those of active microbial communities. As the latter may differently influence metal accumulation in A. halleri, future studies should evaluate both, DNA and RNA, and utilize RNA:DNA ratios as a proxy for microbial activity (Mei et al., 2016; Bowsher et al., 2019; Honeker et al., 2019). Similarly, assessing the bioavailable fractions of a larger suit of elements may identify additional relevant variables driving metal accumulation in plants.
5. CONCLUSIONS
This study provides new insight into the association of soil microbial populations with Zn and Cd hyperaccumulation traits in A. halleri growing at M and NM sites. Metal contamination significantly altered the structure of soil bacterial/archaeal and fungal communities and influenced the number of unique taxa recruited by the A. halleri rhizosphere. Additionally, results show that Zn hyperaccumulation was predominantly governed by biotic variables, whereas variability in Cd hyperaccumulation was primarily explained by abiotic factors. We have identified a group of microbial taxa that consistently associated with Zn hyperaccumulation by A. halleri, regardless of soil metal contamination levels. These findings suggest that these taxa not only increase metal mobilization in the soil and hyperaccumulation by A. halleri, but also benefit overall plant performance. This can result in hyperaccumulation of Zn even in NM sites such as NM_PL14. The identification of taxa with potential to support metal hyperaccumulation is important from an applied perspective – it is a necessary step towards optimizing phytoextraction of metals from soil. We highlight the importance of selecting the most promising hyperaccumulating plant populations and the need to design highly specific microbial inocula with distinct functional properties to aid in the hyperaccumulation process. Future work should focus on: i) unraveling the functional role of the herein identified microorganisms for plant metal hyperaccumulation and ii) assessing the effective role of soil abiotic and biotic factors in the adaptive evolution of A. halleri at M and NM sites, e.g., through reciprocal transplant experiments.
Supplementary Material
HIGHLIGHTS.
Key drivers of metal hyperaccumulation variability in plants are not fully understood
A. halleri rhizosphere recruits more unique microbial taxa at contaminated sites
Zn hyperaccumulation in A. halleri associates with rhizosphere microbial communities
Cd hyperaccumulation in A. halleri is governed by abiotic soil parameters
Locally optimized combinations of plants and microbes will enhance phytoremediation
ACKNOWLEDGEMENTS
This work was supported by the MINIATURA2 grant financed by the National Science Centre, Poland (2018/02/X/NZ8/00546), the POWROTY/REINTEGRATION programme of the Foundation for Polish Science cofinanced by the European Union under the European Regional Development Fund (POIR.04.04.00-00-1D79/16-01), and by the National Institute of Environmental and Health Sciences Superfund Research Program (Grant P42ES04940) at the University of Arizona.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
REFERENCES
- Ali H, Khan E, Sajad MA. 2013. Phytoremediation of heavy metals—concepts and applications. Chemosphere 91(7): 869–881. [DOI] [PubMed] [Google Scholar]
- Anandham R, Indira Gandhi P, Madhaiyan M, Sa T. 2008. Potential plant growth promoting traits and bioacidulation of rock phosphate by thiosulfate oxidizing bacteria isolated from crop plants. Journal of Basic Microbiology 48(6): 439–447. [DOI] [PubMed] [Google Scholar]
- Anderson MJ. 2001. A new method for non-parametric multivariate analysis of variance. Austral Ecology 26(1): 32–46. [Google Scholar]
- Asad SA, Farooq M, Afzal A, West H. 2019. Integrated phytobial heavy metal remediation strategies for a sustainable clean environment-a review. Chemosphere 217: 925–941. [DOI] [PubMed] [Google Scholar]
- Azarbad H, Niklińska M, Laskowski R, van Straalen NM, van Gestel CAM, Zhou J, He Z, Wen C, Röling WFM. 2015. Microbial community composition and functions are resilient to metal pollution along two forest soil gradients. FEMS Microbiology Ecology 91(1): 1–11. [DOI] [PubMed] [Google Scholar]
- Babst-Kostecka A, Schat H, Saumitou-Laprade P, Grodzińska K, Bourceaux A, Pauwels M, Frérot H. 2018. Evolutionary dynamics of quantitative variation in an adaptive trait at the regional scale: The case of zinc hyperaccumulation in Arabidopsis halleri. Molecular Ecology 27(16): 3257–3273. [DOI] [PubMed] [Google Scholar]
- Baker AJM. 1981. Accumulators and Excluders - Strategies in the response of Plants to heavy metals. Journal of Plant Nutrition 3(1–4): 643–654. [Google Scholar]
- Baker AJM. 1987. Metal tolerance. New Phytologist 106(suppl.): 93–111. [Google Scholar]
- Balabane M, Faivre D, van Oort F, Dahmani-Muller H. 1999. Mutual effects of soil organic matter dynamics and heavy metals fate in a metallophyte grassland. Environmental Pollution 105(1): 45–54. [Google Scholar]
- Bamborough L, Cummings SP. 2009. The impact of increasing heavy metal stress on the diversity and structure of the bacterial and actinobacterial communities of metallophytic grassland soil. Biology and Fertility of Soils 45(3): 273–280. [Google Scholar]
- Bartholdy BA, Berreck M, Haselwandter K. 2001. Hydroxamate siderophore synthesis by Phialocephala fortinii, a typical dark septate fungal root endophyte. BioMetals 14(1): 33–42. [DOI] [PubMed] [Google Scholar]
- Barton CJ. 1948. Photometric analysis of phosphate rock. Analytical Chemistry 20(11): 1068–1073. [Google Scholar]
- Bell TH, Cloutier-Hurteau B, Al-Otaibi F, Turmel MC, Yergeau E, Courchesne F, St-Arnaud M. 2015. Early rhizosphere microbiome composition is related to the growth and Zn uptake of willows introduced to a former landfill. Environmental Microbiology 17(8): 3025–3038. [DOI] [PubMed] [Google Scholar]
- Berg J, Brandt KK, Al-Soud WA, Holm PE, Hansen LH, Sørensen SJ, Nybroe O. 2012. Selection for Cu-tolerant bacterial communities with altered composition, but unaltered richness, via long-term cu exposure. Applied and Environmental Microbiology 78(20): 7438–7446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bert V, Bonnin I, Saumitou-Laprade P, de Laguérie P, Petit D. 2002. Do Arabidopsis halleri from nonmetalicolous populations accumulate zinc and cadmium more effectively than those from metallicolous populations? New Phytologist 155: 47–57. [DOI] [PubMed] [Google Scholar]
- Bert V, Macnair MR, de Laguerie P, Saumitou-Laprade P, Petit D. 2000. Zinc tolerance and accumulation in metallicolous and non-metallicolous populations of Arabidopsis halleri (Brassicaceae). New Phytologist 146: 225–233. [DOI] [PubMed] [Google Scholar]
- Bowsher AW, Kearns PJ, Shade A. 2019. 16S rRNA/rRNA gene ratios and cell activity staining reveal consistent patterns of microbial activity in plant-associated soil. mSystems 4(2): e00003–00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bressan M, Roncato MA, Bellvert F, Comte G, Haichar FEZ, Achouak W, Berge O. 2009. Exogenous glucosinolate produced by Arabidopsis thaliana has an impact on microbes in the rhizosphere and plant roots. ISME Journal 3(11): 1243–1257. [DOI] [PubMed] [Google Scholar]
- Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJA, Holmes SP. 2016. DADA2: high-resolution sample inference from illumina amplicon data. Nat Methods 13(7): 581–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao X, Luo J, Wang X, Chen Z, Liu G, Khan MB, Kang KJ, Feng Y, He Z, Yang X. 2020. Responses of soil bacterial community and Cd phytoextraction to a Sedum alfredii-oilseed rape (Brassica napus L. and Brassica juncea L.) intercropping system. Science of the Total Environment 723: 138152. [DOI] [PubMed] [Google Scholar]
- Carrascal LM, Galván I, Gordo O. 2009. Partial least squares regression as an alternative to current regression methods used in ecology. Oikos 118(5): 681–690. [Google Scholar]
- Corso M, Schvartzman MS, Guzzo F, Souard F, Malkowski E, Hanikenne M, Verbruggen N. 2018. Contrasting cadmium resistance strategies in two metallicolous populations of Arabidopsis halleri. New Phytologist 218: 283–297. [DOI] [PubMed] [Google Scholar]
- Dąbrowska G, Hrynkiewicz K, Trejgell A, Baum C. 2017. The effect of plant growth-promoting rhizobacteria on the phytoextraction of Cd and Zn by Brassica napus L. International journal of phytoremediation 19(7): 597–604. [DOI] [PubMed] [Google Scholar]
- De Maria S, Rivelli AR, Kuffner M, Sessitsch A, Wenzel WW, Gorfer M, Strauss J, Puschenreiter M. 2011. Interactions between accumulation of trace elements and macronutrients in Salix caprea after inoculation with rhizosphere microorganisms. Chemosphere 84(9): 1256–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell’Amico E, Cavalca L, Andreoni V. 2008. Improvement of Brassica napus growth under cadmium stress by cadmium-resistant rhizobacteria. Soil Biology and Biochemistry 40(1): 74–84. [Google Scholar]
- Dietrich CC, Bilnicki K, Korzeniak U, Briese C, Nagel KA, Babst-Kostecka A. 2019. Does slow and steady win the race? Root growth dynamics of Arabidopsis halleri ecotypes in soils with varying trace metal element contamination. Environmental and Experimental Botany 167: 103862. [Google Scholar]
- Dietrich CC, Tandy S, Banaś A, Murawska K, Korzeniak U, Łopata B, Babst-Kostecka A. 2021. Phytoextraction efficiency of Arabidopsis halleri is driven by the plant and not by soil metal concentration. Chemosphere: 131437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farinati S, DalCorso G, Bona E, Corbella M, Lampis S, Cecconi D, Polati R, Berta G, Vallini G, Furini A. 2009. Proteomic analysis of Arabidopsis halleri shoots in response to the heavy metals cadmium and zinc and rhizosphere microorganisms. Proteomics 9(21): 4837–4850. [DOI] [PubMed] [Google Scholar]
- Farinati S, DalCorso G, Panigati M, Furini A. 2011. Interaction between selected bacterial strains and Arabidopsis halleri modulates shoot proteome and cadmium and zinc accumulation. Journal of experimental botany 62(10): 3433–3447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrés M, Platikanov S, Tsakovski S, Tauler R. 2015. Comparison of the variable importance in projection (VIP) and of the selectivity ratio (SR) methods for variable selection and interpretation. Journal of Chemometrics 29(10): 528–536. [Google Scholar]
- Frérot H, Hautekèete N-C, Decombeix I, Bouchet M-H, Créach A, Saumitou-Laprade P, Piquot Y, Pauwels M. 2018. Habitat heterogeneity in the pseudometallophyte Arabidopsis halleri and its structuring effect on natural variation of zinc and cadmium hyperaccumulation. Plant and soil 423: 157–174. [Google Scholar]
- Gans J, Wolinsky M, Dunbar J. 2005. Computational improvements reveal great bacterial diversity and high metal toxicity in soil. Science 309(5739): 1387–1390. [DOI] [PubMed] [Google Scholar]
- Honeker LK, Gullo CF, Neilson JW, Chorover J, Maier RM. 2019. Effect of Re-acidification on Buffalo Grass Rhizosphere and Bulk Microbial Communities During Phytostabilization of Metalliferous Mine Tailings. Frontiers in Microbiology 10(1209). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honjo MN, Kudoh H. 2019. Arabidopsis halleri: a perennial model system for studying population differentiation and local adaptation. AoB Plants 11(6): plz076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W, Schoenau J. 1998. Fluxes of water-soluble nitrogen and phosphorus in the forest floor and surface mineral soil of a boreal aspen stand. Geoderma 81(3–4): 251–264. [Google Scholar]
- Khan S, Hesham AEL, Qiao M, Rehman S, He JZ. 2010. Effects of Cd and Pb on soil microbial community structure and activities. Environmental Science and Pollution Research 17(2): 288–296. [DOI] [PubMed] [Google Scholar]
- Kuffner M, Puschenreiter M, Wieshammer G, Gorfer M, Sessitsch A. 2008. Rhizosphere bacteria affect growth and metal uptake of heavy metal accumulating willows. Plant and soil 304(1): 35–44. [Google Scholar]
- Kushwaha P, Neilson JW, Barberán A, Chen Y, Fontana CG, Butterfield BJ, Maier RM. 2021. Arid Ecosystem Vegetation Canopy-Gap Dichotomy: Influence on Soil Microbial Composition and Nutrient Cycling Functional Potential. Applied and Environmental Microbiology 87(5): 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laranjo M, Alexandre A, Oliveira S. 2014. Legume growth-promoting rhizobia: An overview on the Mesorhizobium genus. Microbiological Research 169(1): 2–17. [DOI] [PubMed] [Google Scholar]
- Li WC, Ye ZH, Wong MH. 2007. Effects of bacteria on enhanced metal uptake of the Cd/Zn-hyperaccumulating plant, Sedum alfredii. Journal of experimental botany 58(15–16): 4173–4182. [DOI] [PubMed] [Google Scholar]
- Lodewyckx C, Mergeay M, Vangronsveld J, Clijsters H, Van Der Lelie D. 2002. Isolation, characterization, and identification of bacteria associated with the zinc hyperaccumulator Thlaspi caerulescens subsp. calaminaria. International journal of phytoremediation 4(2): 101–115. [DOI] [PubMed] [Google Scholar]
- Lopez S, Piutti S, Vallance J, Morel J-L, Echevarria G, Benizri E. 2017. Nickel drives bacterial community diversity in the rhizosphere of the hyperaccumulator Alyssum murale. Soil Biology and Biochemistry 114: 121–130. [Google Scholar]
- Luo Y, Wu Y, Wang H, Xing R, Zheng Z, Qiu J, Yang L. 2018. Bacterial community structure and diversity responses to the direct revegetation of an artisanal zinc smelting slag after 5 years. Environmental Science and Pollution Research 25(15): 14773–14788. [DOI] [PubMed] [Google Scholar]
- Luo ZB, Wu C, Zhang C, Li H, Lipka U, Polle A. 2014. The role of ectomycorrhizas in heavy metal stress tolerance of host plants. Environmental and Experimental Botany 108: 47–62. [Google Scholar]
- Ma Y, Oliveira RS, Nai F, Rajkumar M, Luo Y, Rocha I, Freitas H. 2015. The hyperaccumulator Sedum plumbizincicola harbors metal-resistant endophytic bacteria that improve its phytoextraction capacity in multi-metal contaminated soil. Journal of Environmental Management 156: 62–69. [DOI] [PubMed] [Google Scholar]
- Markert JA, Champlin DM, Gutjahr-Gobell R, Grear JS, Kuhn A, McGreevy TJ, Roth A, Bagley MJ, Nacci DE. 2010. Population genetic diversity and fitness in multiple environments. BMC evolutionary biology 10(1): 205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei R, Narihiro T, Nobu M, Liu WT. 2016. Effects of heat shocks on microbial community structure and microbial activity of a methanogenic enrichment degrading benzoate. Letters in applied microbiology 63(5): 356–362. [DOI] [PubMed] [Google Scholar]
- Mendes R, Garbeva P, Raaijmakers JM. 2013. The rhizosphere microbiome: significance of plant beneficial, plant pathogenic, and human pathogenic microorganisms. FEMS microbiology reviews 37(5): 634–663. [DOI] [PubMed] [Google Scholar]
- Mendez MO, Maier RM. 2008. Phytostabilization of mine tailings in arid and semiarid environments - An emerging remediation technology. Environmental Health Perspectives 116(3): 278–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer C-L, Kostecka AA, Saumitou-Laprade P, Créach A, Castric V, Pauwels M, Frérot H. 2010. Variability of zinc tolerance among and within populations of the pseudometallophyte Arabidopsis halleri and possible role of directional selection. New Phytologist 185(1): 130–142. [DOI] [PubMed] [Google Scholar]
- Micallef SA, Shiaris MP, Colón-Carmona A. 2009. Influence of Arabidopsis thaliana accessions on rhizobacterial communities and natural variation in root exudates. Journal of experimental botany 60(6): 1729–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miethling R, Wieland G, Backhaus H, Tebbe CC. 2000. Variation of microbial rhizosphere communities in response to crop species, soil origin, and inoculation with Sinorhizobium meliloti L33. Microbial Ecology 40(1): 43–56. [DOI] [PubMed] [Google Scholar]
- Miransari M. 2010. Contribution of arbuscular mycorrhizal symbiosis to plant growth under different types of soil stress. Plant Biology 12(4): 563–569. [DOI] [PubMed] [Google Scholar]
- Miransari M. 2011. Hyperaccumulators, arbuscular mycorrhizal fungi and stress of heavy metals. Biotechnology Advances 29(6): 645–653. [DOI] [PubMed] [Google Scholar]
- Muehe EM, Weigold P, Adaktylou IJ, Planer-Friedrich B, Kraemer U, Kappler A, Behrens S. 2015. Rhizosphere microbial community composition affects cadmium and zinc uptake by the metal-hyperaccumulating plant Arabidopsis halleri. Applied and Environmental Microbiology 81(6): 2173–2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson RH, Larsson K-H, Taylor AFS, Bengtsson-Palme J, Jeppesen TS, Schigel D, Kennedy P, Picard K, Gï Ockner FO, Tedersoo L, et al. 2018. The UNITE database for molecular identification of fungi: handling dark taxa and parallel taxonomic classifications. Nucleic Acid Res 47(D1): D259–D264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oksanen J, Kindt R, Legendre P, O’Hara B, Simpson GL, Solymos P, Stevens MHM, Wagner H. 2008. The vegan package. Community Ecology Package 10: 631–637. [Google Scholar]
- Patten CL, Glick BR. 2002. Role of Pseudomonas putida indoleacetic acid in development of the host plant root system. Applied and Environmental Microbiology 68(8): 3795–3801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulson JN, Pop M, Bravo HC. 2013. metagenomeSeq: Statistical analysis for sparse high-throughput sequencing. Bioconductor package 1: 191–191. [Google Scholar]
- Penrose DM, Glick BR. 2001. Levels of ACC and related compounds in exudate and extracts of canola seeds treated with ACC deaminase-containing plant growth-promoting bacteria. Canadian Journal of Microbiology 47(4): 368–372. [DOI] [PubMed] [Google Scholar]
- Press CM, Loper JE, Kloepper JW. 2001. Role of iron in rhizobacteria-mediated induced systemic resistance of cucumber. Phytopathology 91(6): 593–598. [DOI] [PubMed] [Google Scholar]
- Quast C, Pruesse E, Yilmaz P, Gerken J, Schweer T, Yarza P, Rg Peplies J, Glö Ckner FO. 2013. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res 41(D1): D590–D596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajapaksha RMCP, Tobor-Kapłon MA, Bååth E. 2004. Metal toxicity affects fungal and bacterial activities in soil differently. Applied and Environmental Microbiology 70(5): 2966–2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves RD, Baker AJM 2000. Metal accumulating plants. In: Raskin I, Ensley BD eds. Phytoremediation of toxic metals: using plants to clean up the environment. New York: Wiley and Sons, 193–229. [Google Scholar]
- Reimann C, Albanese S, Batista M, Bel Lan A, Birke M, Cicchella D, Demetriades A, De Vivo B, De Vos W, Dinelli E. 2008. EuroGeoSurveys Geochemical mapping of agricultural and grazing land soil of Europe (GEMAS)-Field manual. NGU-rapport. [Google Scholar]
- Roosens NH, Willems G, Saumitou-Laprade P. 2008. Using Arabidopsis to explore zinc tolerance and hyperaccumulation. Trends in Plant Science 13(5): 208–215. [DOI] [PubMed] [Google Scholar]
- Rosatto S, Roccotiello E, Di Piazza S, Cecchi G, Greco G, Zotti M, Vezzulli L, Mariotti M. 2019. Rhizosphere response to nickel in a facultative hyperaccumulator. Chemosphere 232: 243–243. [DOI] [PubMed] [Google Scholar]
- Sanchez G, Sanchez MG. 2012. Package ‘plsdepot’. Partial Least Squares (PLS) Data Analysis Methods, v. 0.1 17. [Google Scholar]
- Segata N, Izard J, Waldron L, Gevers D, Miropolsky L, Garrett WS, Huttenhower C. 2011. Metagenomic biomarker discovery and explanation. Genome Biology 12(6): 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng X-F, Xia J-J. 2006. Improvement of rape (Brassica napus) plant growth and cadmium uptake by cadmium-resistant bacteria. Chemosphere 64(6): 1036–1042. [DOI] [PubMed] [Google Scholar]
- Shentu JL, He ZL, Zeng YY, He SY, Du ST, Shen DS. 2014. Microbial biomass and PLFA profile changes in rhizosphere of pakchoi (Brassica chinensis L.) as affected by external cadmium loading. Pedosphere 24(4): 553–562. [Google Scholar]
- Solís-Dominguez FA, White SA, Hutter TB, Amistadi MK, Root RA, Chorover J, Maier RM. 2012. Response of key soil parameters during compost-assisted phytostabilization in extremely acidic tailings: effect of plant species. Environmental science & technology 46(2): 1019–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto J, Ortiz J, Herrera H, Fuentes A, Almonacid L, Charles TC, Arriagada C. 2019. Enhanced arsenic tolerance in Triticum aestivum inoculated with arsenic-resistant and plant growth promoter microorganisms from a heavy metal-polluted soil. Microorganisms 7(9): 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein RJ, Höreth S, de Melo JRF, Syllwasschy L, Lee G, Garbin ML, Clemens S, Krämer U. 2017. Relationships between soil and leaf mineral composition are element-specific, environment-dependent and geographically structured in the emerging model Arabidopsis halleri. New Phytologist 213(3): 1274–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun M, Xiao T, Ning Z, Xiao E, Sun W. 2015. Microbial community analysis in rice paddy soils irrigated by acid mine drainage contaminated water. Applied Microbiology and Biotechnology 99(6): 2911–2922. [DOI] [PubMed] [Google Scholar]
- Swanner E, Templeton A. 2011. Potential for nitrogen fixation and nitrification in the granite-hosted subsurface at Henderson Mine, CO. Frontiers in Microbiology, 2, 254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talke IN, Hanikenne M, Krämer U. 2006. Zinc-dependent Global Transcriptional Control, Transcriptional Deregulation, and Higher Gene Copy Numer for Genes in Metal Homeostasis of the Hyperaccumulator Arabidopsis halleri. Plant Physiology 142: 148–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thijs S, Langill T, Vangronsveld J 2017. The bacterial and fungal microbiota of hyperaccumulator plants: Small organisms, large influence. Advances in Botanical Research: Elsevier, 43–86. [Google Scholar]
- Tipayno SC, Truu J, Samaddar S, Truu M, Preem JK, Oopkaup K, Espenberg M, Chatterjee P, Kang Y, Kim K, et al. 2018. The bacterial community structure and functional profile in the heavy metal contaminated paddy soils, surrounding a nonferrous smelter in South Korea. Ecology and Evolution 8(12): 6157–6168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi P, Leach JE, Tringe SG, Sa T, Singh BK. 2020. Plant–microbiome interactions: from community assembly to plant health. Nature reviews microbiology 18(11): 607–621. [DOI] [PubMed] [Google Scholar]
- Valentín-Vargas A, Neilson JW, Root RA, Chorover J, Maier RM. 2018. Treatment impacts on temporal microbial community dynamics during phytostabilization of acid-generating mine tailings in semiarid regions. Science of the Total Environment 618: 357–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Ent A, Baker AJM, Reeves RD, Pollard AJ, Schat H. 2013. Hyperaccumulators of metal and metalloid trace elements: Facts and fiction. Plant and soil 362(1–2): 319–334. [Google Scholar]
- Virgone K, Ramirez-Andreotta M, Mainhagu J, Brusseau ML. 2018. Effective integrated frameworks for assessing mining sustainability. Environmental Geochemistry and Health 40(6): 2635–2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters W, Hyde ER, Berg-Lyons D, Ackermann G, Humphrey G, Parada A, Gilbert JA, Jansson JK, Gregory Caporaso J, Fuhrman JA, et al. 2016. Improved bacterial 16S rRNA gene (V4 and V4–5) and fungal internal transcribed spacer marker gene primers for microbial community surveys. mSystems 1(1): e00009–00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao X, Fan M, Wang E, Chen W, Wei G. 2017. Interactions of plant growth-promoting rhizobacteria and soil factors in two leguminous plants. Applied Microbiology and Biotechnology 101(23–24): 8485–8497. [DOI] [PubMed] [Google Scholar]
- Xu Y, Seshadri B, Bolan N, Sarkar B, Ok YS, Zhang W, Rumpel C, Sparks D, Farrell M, Hall T, et al. 2019. Microbial functional diversity and carbon use feedback in soils as affected by heavy metals. Environment International 125(February): 478–488. [DOI] [PubMed] [Google Scholar]
- Yang S, Zhang X, Cao Z, Zhao K, Wang S, Chen M, Hu X. 2014. Growth-promoting sphingomonas paucimobilis ZJSH1 associated with Dendrobium officinale through phytohormone production and nitrogen fixation. Microbial Biotechnology 7(6): 611–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeoh YK, Dennis PG, Paungfoo-Lonhienne C, Weber L, Brackin R, Ragan MA, Schmidt S, Hugenholtz P. 2017. Evolutionary conservation of a core root microbiome across plant phyla along a tropical soil chronosequence. Nature Communications 8(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarei M, Hempel S, Wubet T, Schäfer T, Savaghebi G, Jouzani GS, Nekouei MK, Buscot F. 2010. Molecular diversity of arbuscular mycorrhizal fungi in relation to soil chemical properties and heavy metal contamination. Environmental Pollution 158(8): 2757–2765. [DOI] [PubMed] [Google Scholar]
- Zhu B, Zhang X, Zhao C, Chen S, Yang S. 2018. Comparative genome analysis of marine purple sulfur bacterium Marichromatium gracile YL28 reveals the diverse nitrogen cycle mechanisms and habitat-specific traits. Scientific reports 8(1): 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


