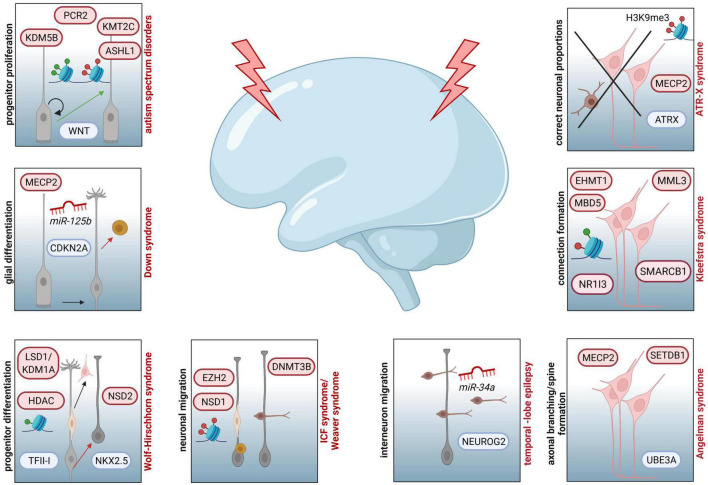
FIGURE 2.
Examples of NDDs involving epigenetic key players and affected processes during corticogenesis assumed to contribute to the respective diseases. Increased progenitor proliferation and decreased neurogenesis due to dysregulated function of WNT can be traced back to aberrant activity of PCR2, KMT2C, KDM5B or ASHL1 in ASD. In individuals affected by Down syndrome, miR125b and MECP2 are associated to altered CDKN2A expression and glial proliferation. For patients with Wolf-Hirschhorn syndrome neuronal progenitor proliferation is disturbed by aberrant activity of LSD1/KDM1A and HDAC, both targeting the transcription factor TFII-I, as well as dysregulated function of NSD2, which is influencing the expression of NKX2.5. In case of ICF syndrome, associated mutations in DNMT3B are resulting in hypomethylated genes essential for neuronal migration (LHX2, ROBO1, CXCR4, IFRD2, DTX4, ENC1, JARID2, SEMA3B, and ITM2). Additionally, patients with Weaver syndrome are characterized by neuronal migration defects, due to haploinsufficiency of EZH2, disturbing PRC2 activity, and mutations in NSD1, impairing proper establishment of histone marks. At the level of interneuron migration, different miRNAs are regulating this pivotal step of corticogenesis. For example, downregulation of miR-34a is affecting NEUROG2 expression, which is essential for neuronal migration. Mutations in UBE3A and dysregulated expression of MECP2 and SETDB1 are linked to susceptibility of Angelman syndrome, which is also characterized by deficits in axonal branching, spine formation and synapse generation. In individuals with Kleefstra syndrome, dysregulated interhemispheric connections are reported as potential result of mutations in EHMT1, leading to disturbed interaction with EHMT2 or EZH2 and changes in expression of genes coding for epigenetic regulators, such as MML3, SMARCB1, NR1I3, or MBD5. Deficits in ATRX-MECP2 interaction, subsequent aberrations in H3K9me3 marks and resulting improper neuronal proportions in different cortical and subcortical areas are depicted for ATR-X syndrome.