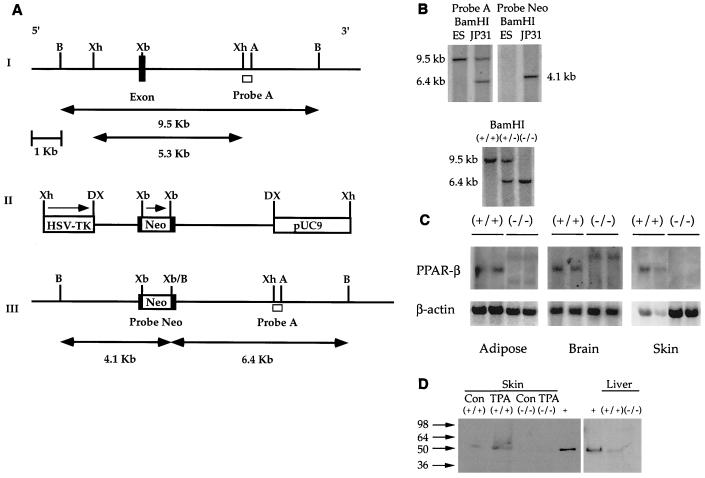
FIG. 1.
Targeted disruption of the mPPARβ gene. (A) Strategy for the mPPARβ knockout. (I) Partial map of a mouse genomic fragment containing the second-to-last and last exons encoding the mPPARβ ligand binding domain. Restriction enzymes: B, BamHI; Xh, XhoI; Xb, XbaI; A, AflII. The wild-type 9.5-kb BamHI fragment detected by probe A, a 0.65-kb XhoI-AflII fragment from the 3′ end of the mPPARβ genomic DNA, is indicated. (II) Targeting vector with a total of 5.3 kb of homologous sequence contained in the XhoI fragment of the genomic clone. The 1.14-kb NEO gene in the same orientation relative to mPPARβ transcription was inserted into the XbaI site indicated. The NEO cassette introduces a new BamHI restriction site used for genotyping by Southern blot analysis. A pMCITK expression cassette (herpes simplex virus thymidine kinase [HSV-TK]) was added at the 3′ end of the construct for negative selection. DX, disrupted XhoI site. (III) The expected homologous recombination event of mPPARβ. When one allele of the mPPARβ gene was replaced with the targeting vector sequences by homologous recombination, a 6.4-kb restriction fragment appeared when the gene was analyzed with probe A. (B) Genomic Southern blots of ES cell DNA (top) and Southern blot of tail DNA from wild-type (+/+), heterozygous (+/−), and homozygous mutant (−/−) mice (bottom). (C) Northern analysis of PPARβ mRNA in selected tissues from wild-type (+/+) and PPARβ-null (−/−) mice. (D) Western blot analysis of skin and liver from wild-type (+/+) and PPARβ-null (−/−) mice. Samples from skin of TPA-treated mice were also analyzed. +, positive control. Con, control.