Figure 17.
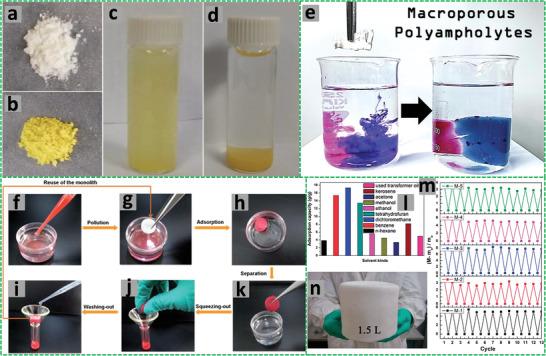
Images show amidoxime‐modified hollow porous melamine resin polymer microspheres before adsorption (a,c) and after adsorption (b,d) of U(VI). Reproduced with permission.[ 165 ] Copyright 2020, Elsevier. e) Photograph shows dyes removal by macroporous amphoteric polyelectrolyte monolith. Reproduced with permission.[ 147 ] Copyright 2020, Elsevier. f) Water polluted with gasoline and dyed with 1,6,7,12‐tetra‐tert‐butylphenoxyperylene‐3,4,9,10‐tetracarboxylic dianhydride, g) addition of porous PS monolith to gasoline mixture, h) adsorption of gasoline by monolith, i) monolith recovery after adsorption, j) pressing of monolith to remove gasoline, and k) washing of monolith for reuse. Reproduced with permission.[ 109 ] Copyright 2013, Royal Society of Chemistry. The graphs show l) adsorption capacities of LMMG‐based W/O gel emulsion‐templated porous polymeric monolith (M‐3), m) regeneration studies of monoliths prepared via different methods (M‐1–M‐5) using kerosene oil as an example, and n) a digital photograph of M‐3 monolith. M‐1–M‐5 indicates monoliths prepared with different silanes. Adapted with permission.[ 181 ] Copyright 2014, Royal Society of Chemistry.
