Figure 3. Effect of Sacubitril/Valsartan and Individualized Medical Therapy on NT-proBNP in Predefined Subgroups.
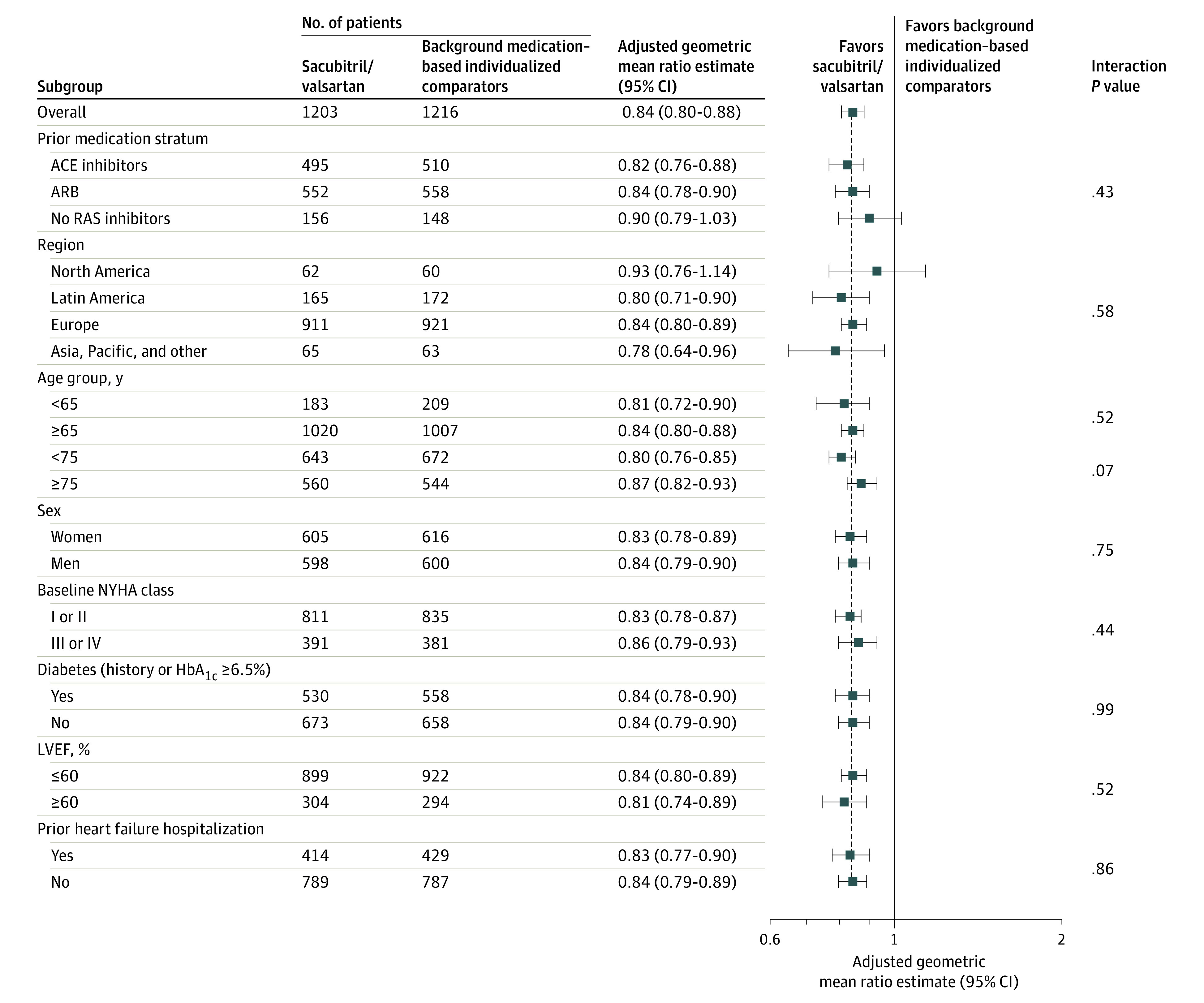
An adjusted geometric mean ratio lower than 1 favors sacubitril/valsartan. The interaction P value is for the subgroup variable × the treatment interaction at week 12. The mixed model for the repeated-measures model includes stratum angiotensin-converting enzyme (ACE) inhibitors, angiotensin II receptor blockers (ARBs), no renin angiotensin system inhibitors, region, treatment (sacubitril/valsartan, background medication–based individualized comparators), visit, treatment × visit interaction, subgroup, subgroup × visit interaction, treatment × subgroup interaction, and treatment × subgroup × visit interaction as fixed-effect factors; baseline log-transformed N-terminal pro–brain natriuretic peptide (NT-proBNP), stratum × baseline log-transformed NT-proBNP, and visit × baseline log-transformed NT-proBNP interactions as covariates; and models the within-patient covariance using an unstructured covariance matrix (a common matrix for the 2 treatment groups). The analysis includes data observed up to week 12. Test values below lower or above the upper limit of quantification are imputed by 0.5 × the lower limit of quantification × 1.5 × upper limit of quantification.
ACE indicates angiotensin-converting enzyme; ARB, angiotensin II blocker; HbA1c, hemoglobin A1c; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; RAS, renin angiotensin system.
