FIGURE 1.
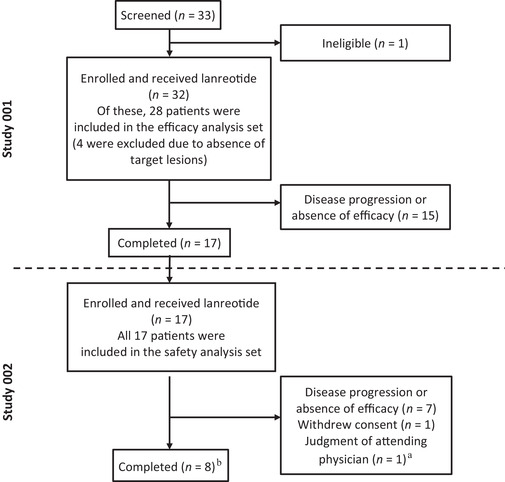
Study design and patient disposition. This open‐label, phase II study of lanreotide in Japanese patients with NETs consisted of two parts: a 48‐week phase II study (Study 001) and its extension study (Study 002). The efficacy analysis set comprised all patients who enrolled in Study 001, received at least one dose of lanreotide and underwent efficacy evaluation at least once. The safety analysis set comprised all patients who completed Study 001 and enrolled in Study 002 and received at least one dose of lanreotide. aThe investigators originally classified one patient as discontinuing treatment because of an adverse event, but finally determined that the adverse event did not warrant discontinuation of the study. bOne patient who completed treatment was not included in the efficacy analysis set as the target tumor site was not evaluable
