Figure 3.
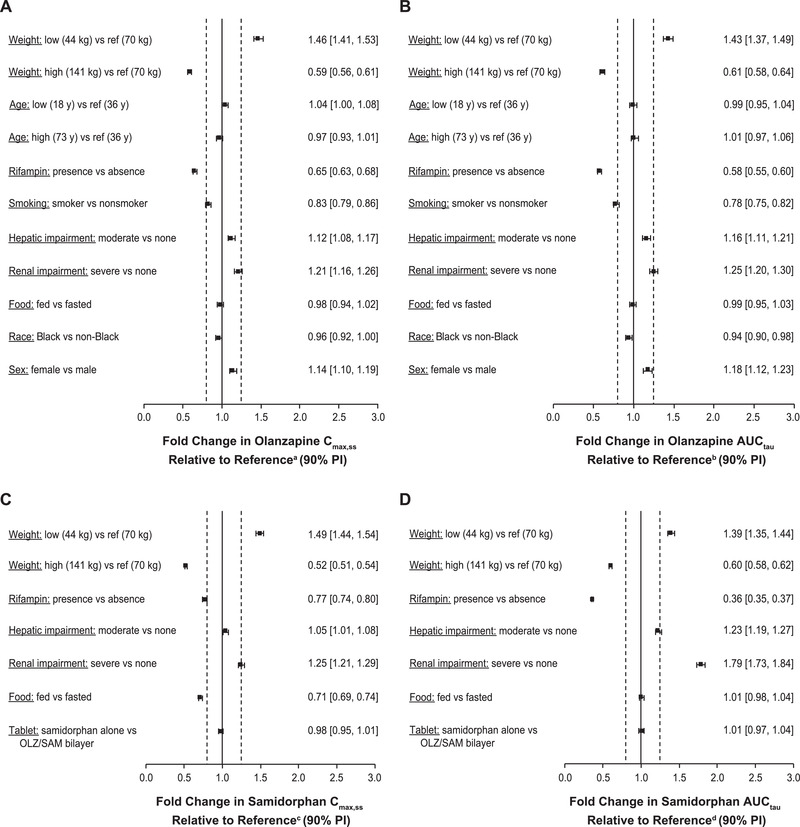
Population pharmacokinetic model–predicted covariate effects on steady‐state (A) olanzapine Cmax,ss, (B) olanzapine AUCtau, (C) samidorphan Cmax,ss, and (D) samidorphan AUCtau.
aReference Cmax,ss for olanzapine is 31.7 ng/mL.
bReference AUCtau for olanzapine is 635 ng · h/mL.
cReference Cmax,ss for samidorphan is 33.4 ng/mL.
dReference AUCtau for samidorphan is 284 ng · h/mL.
The solid vertical line represents no impact of the covariate using a healthy individual with the following characteristics as a reference subject: age, 36 years; weight, 70 kg; non‐Black, nonsmoking man, with normal hepatic and renal function receiving once‐daily oral OLZ/SAM 10 mg/10 mg in a fasted condition. Dashed vertical lines are at 0.8‐ and 1.25‐fold of this value.
AUCtau, area under the plasma concentration‐time curve over the daily dosing interval at steady state; Cmax,ss, maximum concentration at steady state; PI, prediction interval.
