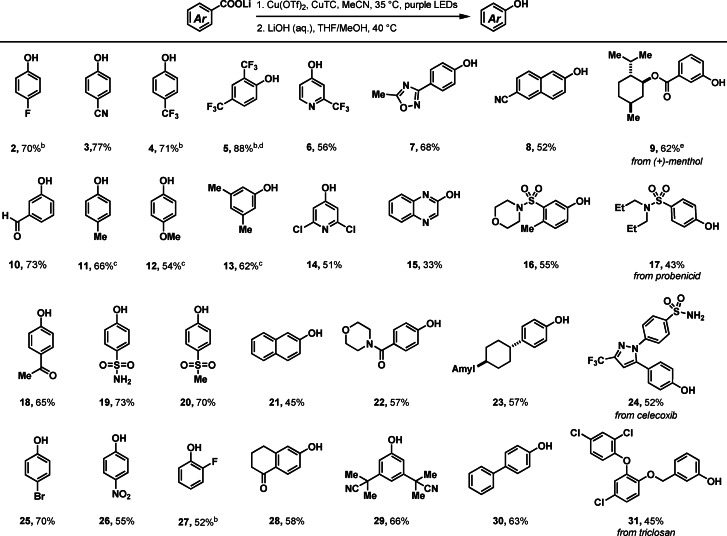
Table 1.
Decarboxylative hydroxylation of benzoic acids.[a]
[a] Standard reaction conditions: 1. lithium carboxylate (0.20 mmol, 1.0 equiv), CuTC (1.5 equiv), Cu(OTf)2 (2.5 equiv), MeCN (c=25 mM), 16 h purple LEDs irradiation, 35 °C; 2. 1 M LiOH (aq.), THF/MeOH (1:1, v/v, 55 mM), 40 °C. [b] Yields based on 19F NMR integration with internal standard 2‐fluorotoluene (0.20 mmol, 1.0 equiv). [c] Yields based on 1H NMR integration with an internal standard dibromomethane (0.20 mmol, 1.0 equiv). [d] Reaction conditions: carboxylic acid (0.20 mmol, 1.0 equiv), LiOH (4 equiv), [Cu(MeCN)4]BF4 (2.5 equiv), Cu(OTf)2 (2.5 equiv), CD3CN (c=50 mM), 16 h purple LEDs irradiation, 35 °C. [e] 2. Aminolyisis: nBuNH2 (2.0 mmol, 10 equiv), benzene (0.10 M), 25 °C.