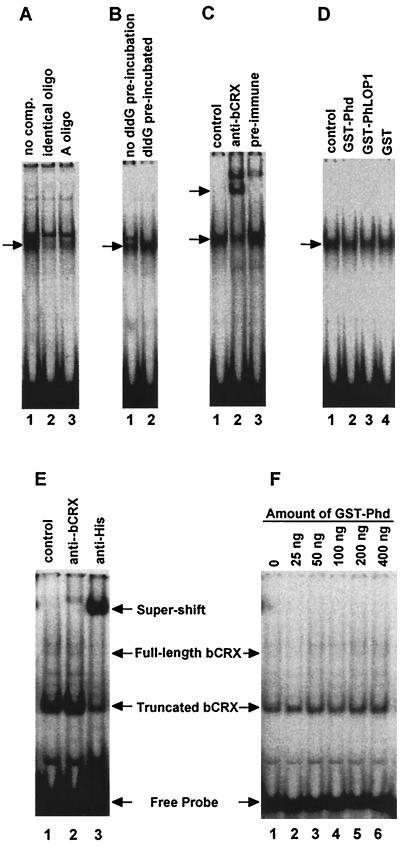
FIG. 7.
EMSA. Equal amounts of a [γ-32P]ATP end-labeled oligonucleotide probe containing both the Ret-1/PCE-1 site and the CRX binding site were incubated with either 2 μg of Weri-Rb-1 cell nuclear extract (A to D) or 50 ng of purified 6xHis-bCRX protein (E and F). Arrows identify specifically shifted or supershifted bands. (A) Lane 1, no competitor; lane 2, 100-fold molar excess of the nonlabeled identical oligonucleotide; lane 3, 100-fold molar excess of the nonlabeled A oligonucleotide. (B) Lane 1, the labeled probe was mixed with poly(dI-dC) before the nuclear extract was added to the reaction mixture; lane 2, poly(dI-dG) was preincubated with the nuclear extract for 10 min before the labeled probe was added. (C) Lane 1, no serum; lane 2, anti-bCRX serum (1:20 final dilution); lane 3, preimmune serum (1:20 final dilution). (D) Fifty nanograms of GST-Phd (lane 2), GST-PhLOP1 (lane 3), or GST (lane 4) purified protein was incubated with 2 μg of Weri-Rb-1 cell nuclear extract before the labeled probe was added. For the control (lane 1), an equal volume of PBS was added to the binding mixture instead of the GST fusion proteins. (E) Lane 1, control; lane 2, affinity-purified anti-bCRX (1:100 final dilution); lane 3, anti-His monoclonal antibody (1:100 final dilution). (F) 6xHis-bCRX (50 ng) was preincubated with increasing amounts of GST-Phd for 10 min before 0.5 fmol of labeled probe (∼5,500 cpm) was added.