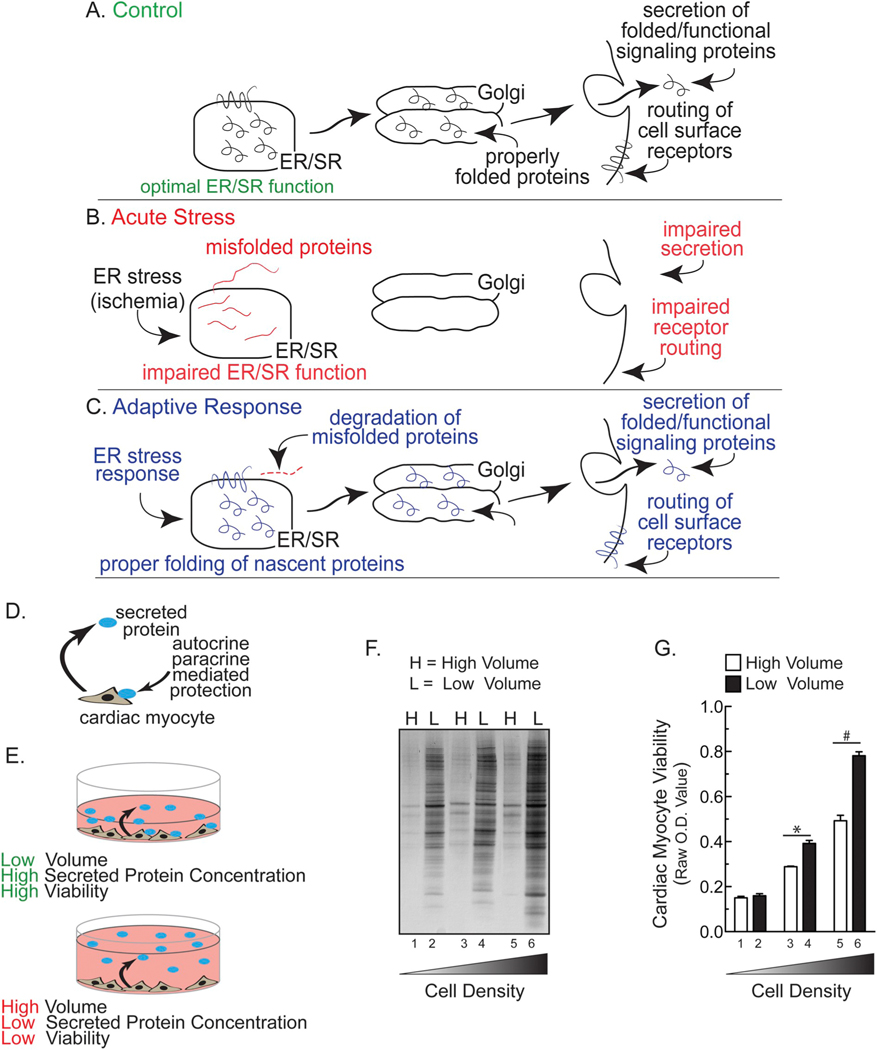
Fig. 1.
Hypothetical Effects of ER Stress on Classical Secretion and Effect of Media Volume and Cell Density on Cardiac Myocyte Viability- Panel A- Proteins secreted by the classical secretory pathway are synthesized and folded in the endoplasmic reticulum (ER), then routed to the Golgi before they are secreted, after which they exert their functions through autocrine, paracrine, and endocrine signaling. Panel B- Hypothesis that acute ER stress impairs ER protein-folding and decreases secretion. Panel C- Hypothesis that in response to ER stress an adaptive response is initiated to reduce misfolded protein accumulation and restore secretion via the classical secretory pathway. Panel D- Diagram describing the hypothesis that secreted proteins could enhance cardiac myocyte viability by autocrine/paracrine-mediated protection. Panel E- Diagram depicting the effects of low (top) or high (bottom) media volumes on auto/paracrine-mediated protection. Panel F- Neonatal rat ventricular myocytes (NRVM) were plated in DMEM/F-12 media containing 10% fetal bovine serum (FBS) at respective densities of 2.5 (lanes 1 and 2), 5.0 (3,4), or 10.0 (5,6) × 105 cells/cm2 in 24-well culture dishes. After 24 h, media were replaced with DMEM/F-12 containing 2% FBS. After another 24 h, media were replaced with either a low volume (L = 0.25 ml; bars 1, 3, 5 in Panel G) or a high volume (H = 2.0 ml; bars 2, 4, 6 in Panel G) of serum-free DMEM/F-12 containing BSA at 1 mg/ml (minimal media). After 48 h, media samples from NRVMs were subjected to SDS-PAGE followed by silver staining. Panel G- Culture viabilities from NRVMs plated as described in Panel F were determined using an MTT assay. Shown are the mean optical density (O.D.) values ± S.E.M. * and #, p ≤ .05 different from paired control.