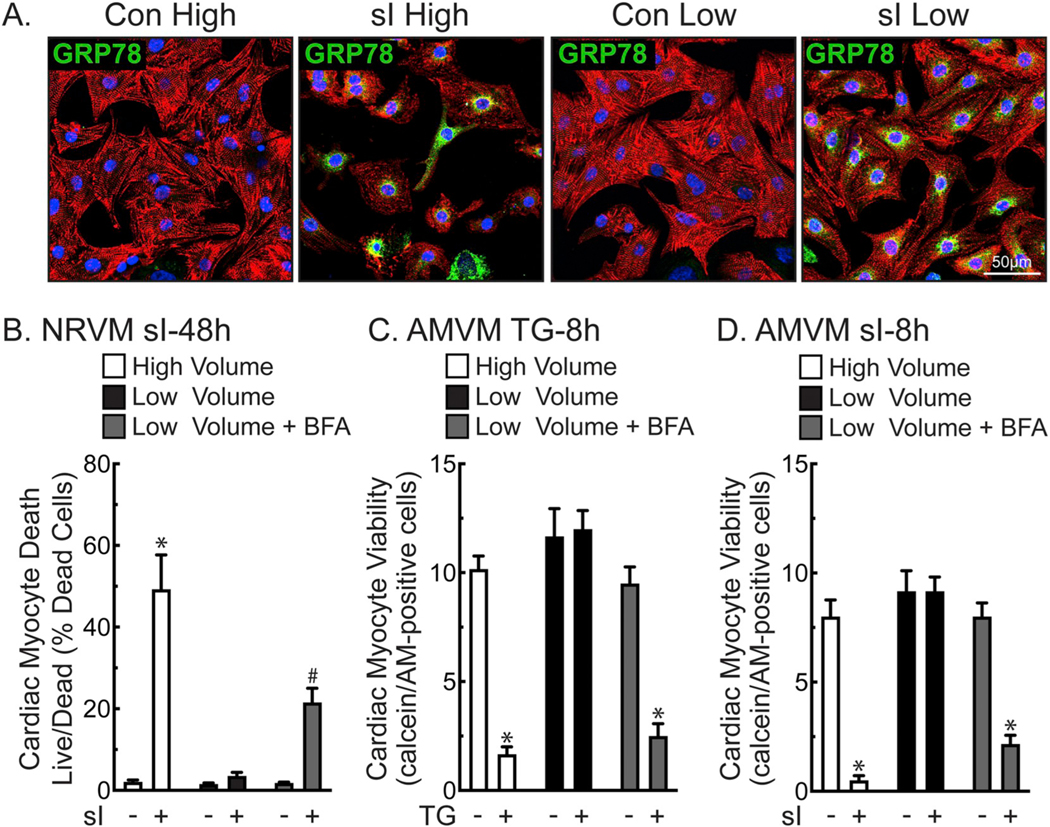
Fig. 3.
Low Volume Protects Cardiac Myocytes from Death during Simulated Ischemia- Panel A- NRVMs plated at 1.1 × 105 cells/chamber on 4-chamber glass slides were treated for 24 h in high (2.5 ml) or low (0.25 ml) media volume with or without simulated ischemia (sI). Cultures were then immunostained for α-actinin (red), GRP78 (green), and stained for DNA (TOPRO-3; blue). Panel B- NRVMs were plated at 4 × 105 cells/12-well culture dish and subjected to sI for 48 h in high or low media volume ± BFA (5 μg/ml), then analyzed for total and dead cells by Hoechst and propidium iodide staining. Shown are the mean values of cell death ± S.E.M., expressed as the percentage of dead cells. *, #, p ≤ .05 different from all other values, as determined by ANOVA followed by Newman Keul’s post hoc analysis. Panels C– Freshly isolated adult mouse ventricular myocytes (AMVM) were plated at 5 × 105 cells/12-well culture dish, then treated for 8 h in high or low maintaining media (see Methods) volume ± TG (2 μm) and BFA (5 μg/ml). AMVMs were scored for viability by quantifying the number of calcein AM-positive, rod-shaped myocytes, as described in the Methods. Shown are the mean values of viable cells ± S.E.M. *, p ≤ .05 different from all other values, as determined by ANOVA followed by Newman Keul’s post hoc analysis. Panel D- AMVMs were plated at 5 × 105 cells/12-well culture dish subjected to sI for 8 h in high or low volume glucose-free DMEM medium containing 2% dialyzed FBS ± BFA (5 μg/ml), and then scored for viability by quantifying the number of calcein AM-positive, rod-shaped myocytes, as described in the Methods. Shown are the mean values of viable cells ± S.E.M. *, p ≤ .05 different from all other values, as determined by ANOVA followed by Newman Keul’s post hoc analysis.