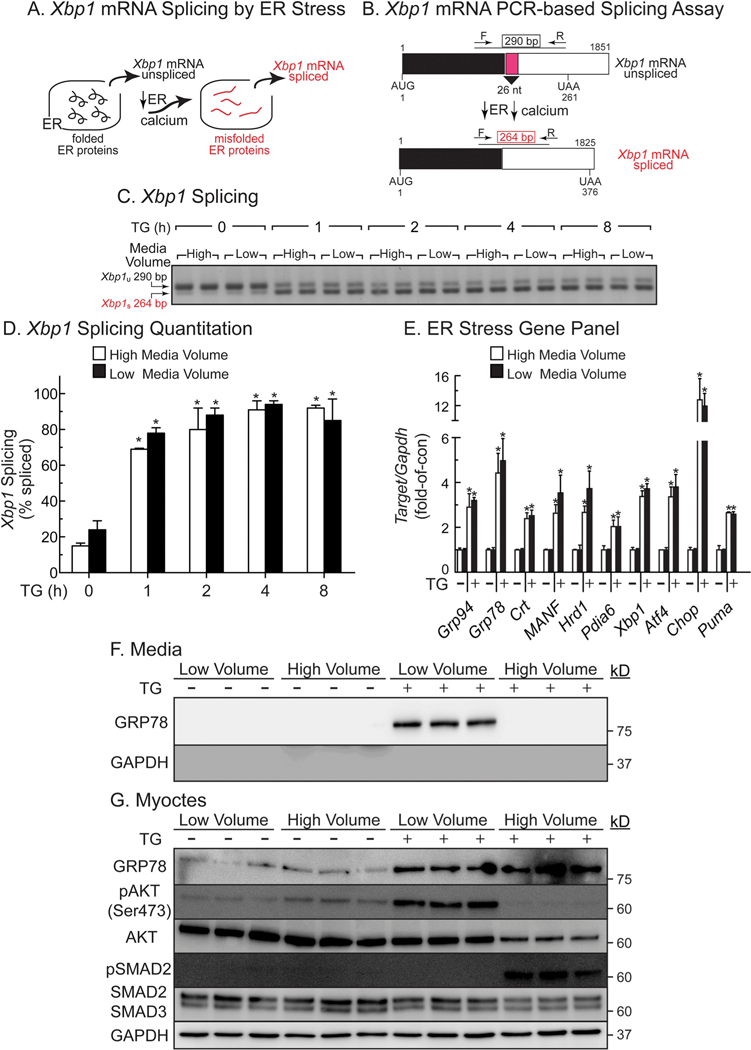
Fig. 4.
Effects of Media Volume and TG on Xbp1 mRNA Splicing and ER Stress Gene Induction- Panel A- Xbp1 splicing is a sensitive measure of protein misfolding in the ER. Panel B- Xbp1 mRNA splicing process and assay. In the absence of ER stress, the Xbp1 mRNA encodes a 261 AA protein that is not a transcription factor. However, upon ER stress, the endoribonuclease activity of IRE-1 is activated, resulting in the removal of a 26-nucleotide intron (pink) and generation of Xbp1 mRNA spliced, which encodes a 376 AA form of Xbp1 that is an active transcription factor. Use of the primers that are shown result in a 290 nucleotide PCR product for the unspliced form of the Xbp1 mRNA and a 264 nucleotide PCR product for the spliced Xbp1 mRNA. Panel C- NRVMs were plated at 1 × 106 cells/6-well culture dish and treated in high (8 ml) or low (1 ml) serum-free DMEM/F-12 containing 1 mg/ml BSA (minimal media) ± TG (2 μM) for the times shown, then extracts were analyzed by PCR for Xbp1 mRNA splicing. Panel D- Quantification of the Xbp1 splicing gel shown in Panel B. *, p ≤ .05 different from untreated control, as determined by ANOVA followed by Newman Keul’s post hoc analysis. Panel E- Gene expression was determined by qRT-PCR using RNA from NRVMs plated at 1 × 106 cells/6-well culture dish and treated for 8 h ± TG (2 μM) in high (8 ml) or low (1 ml) minimal media volumes, as described in Panel B. *, p ≤ .05 different from untreated control, as determined by ANOVA followed by Newman Keul’s post hoc analysis. Panels F, G- NRVMs were plated at 4 × 105 cells/well on 12-well plates. Sixteen hours after plating, cultures were treated for 24 h ± TG (2 μM) in minimal media in low (0.4 ml) or high (2 ml) volume, then examined by SDS-PAGE immunoblotting.