Figure 1.
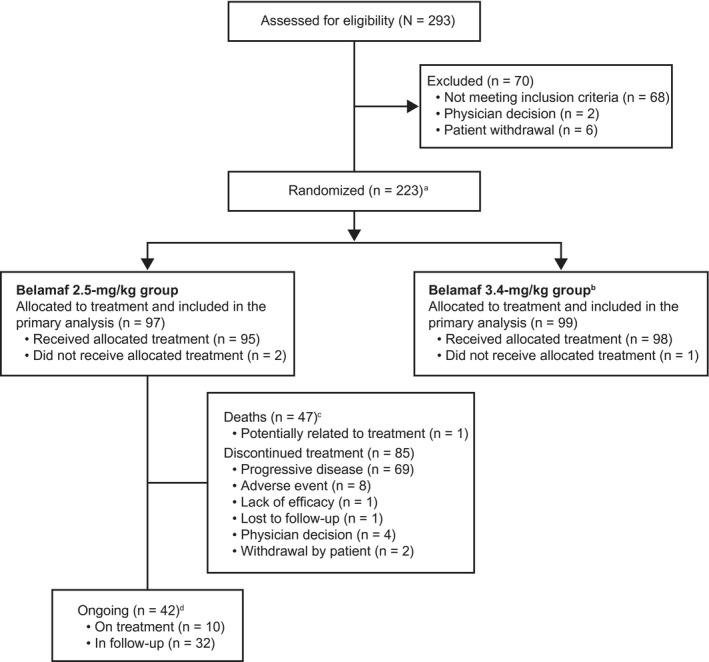
Patient disposition is illustrated. aTwo patients were re‐randomized and counted twice (once per each randomization). An additional independent group of 25 patients was randomized to receive a lyophilized configuration of belantamab mafodotin (belamaf) 3.4 mg/kg and underwent the same assessments and procedures as the main study. This group was analyzed separately from patients who were randomized to receive the frozen solution; as such, the results are not reported here. 28 bPatients who were allocated to receive belamaf 3.4 mg/kg were not included in this analysis and have been reported previously. 16 cPrimary causes of death in the safety population (n = 47) included the disease under study (n = 40), serious adverse event possibly related to study (n = 1; sepsis 16 ), other (n = 3), and unknown cause (n = 3). dA patient would be counted as ongoing with the study if no study conclusion record were included in the disposition data set.
