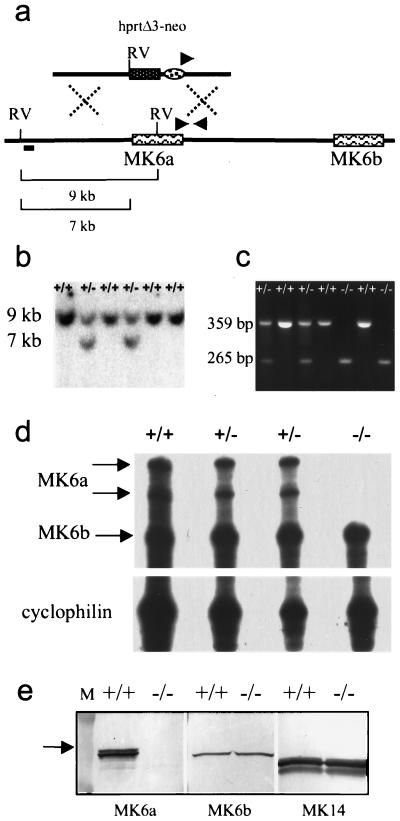
FIG. 1.
Targeting of MK6a. (a) A replacement vector containing the hprtΔ3′-neo cassette was used to delete the MK6a coding region through homologous recombination. RV, EcoRV restriction sites, digestion of which generates a 9-kb fragment for the wild-type locus and a 7-kb fragment for the targeted allele; short bar, position of the 5′ external probe; triangles, positions of the PCR primers used in the 3′ screen. (b) Genomic Southern blot showing the wild-type 9-kb and the targeted 7-kb fragments detected with the 5′ external probe. (c) Genomic PCR of the 3′ end of the targeted locus showing a 359-bp product for the MK6a+/+ genotype, a 265-bp product for MK6a−/−, and both fragments for MK6a+/−. (d) RNase protection assay showing absence of the MK6a transcript in MK6a−/− animals. The RNA was isolated from the 12-O-tetradecanoyl-phorbol-13-acetate-treated ears of littermates. The probe was designed to protect 268 bases in MK6b and 382 bases in MK6a. Note that two bands are generated for the MK6a transcript, most likely due to a polymorphism present in the C57BL/6N strain, resulting in partial degradation of the 382-bp fragment. The probe spans exons three to six, and a single MK6a band was observed in BALB/c mice, the strain from which the cDNA was isolated. A cyclophilin probe was used as a loading control. (e) Western blot analysis of whole skin during anagen showing that MK6a is absent in MK6a−/− mice (left). Note that MK6b is present at lower levels than MK6a and is not upregulated in MK6a−/− mice (middle). MK14 was used as a loading control (right). Note that the doublet bands for MK6a and MK14 most likely are due to differential phosphorylation. Lane M, 84- and 51-kDa size marker bands; arrow, position of the 62.5-kDa band.