FIGURE 3.
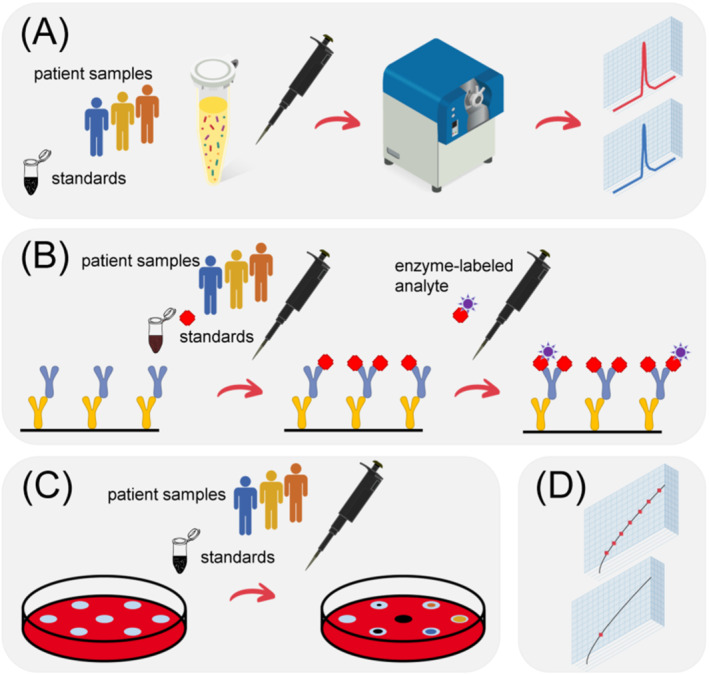
Principles of some of the most‐widely used methods for therapeutic drug monitoring (TDM). (A) Liquid chromatography–mass spectrometry (LC–MS). The target drug is directly measured in plasma and quantified using internal standards. Protein drugs are usually proteolytically digested prior to their quantitation using “proteotypic” peptides. (B) Enzyme‐linked immunosorbent assay (ELISA). In a competitive ELISA, a small molecule drug and an enzyme‐conjugated form of the drug are captured through an immobilized antibody. After washing, the enzyme reaction is used as colorimetric readout: The signal is inversely proportional to the original concentration of the analyte. (C) Bioassay (diffusion). A bacterial strain that is susceptible to the antibiotic to be measured is grown on defined wells in an agar plate. Equal volumes for samples and standards are then added to these wells, and after ca. 24 h of incubation, the diameters of the “inhibition zones” are measured as an indirect readout of the concentration of the antibiotic. (D) Drug concentrations are determined using external or internal calibration curves
