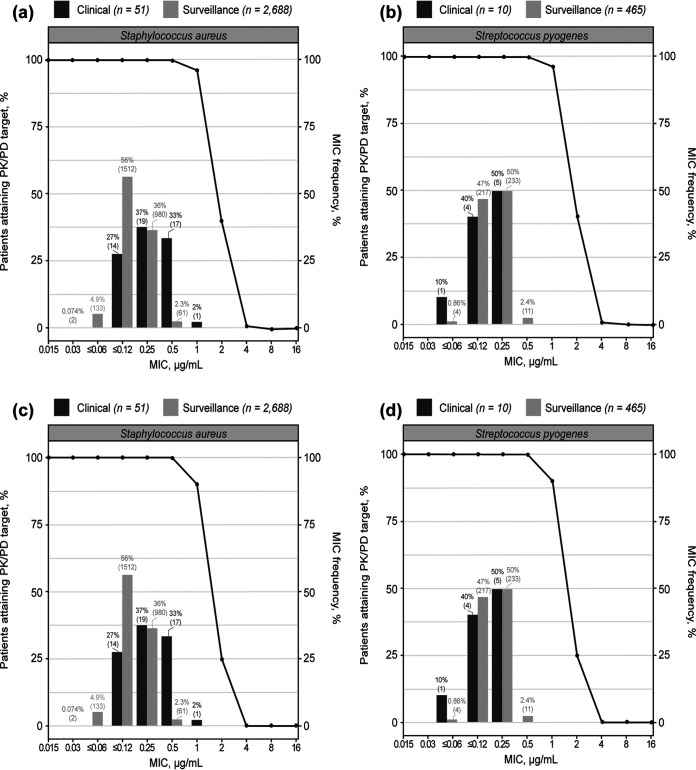
FIG 1.
Proportion of participants achieving tedizolid fAUC/MIC of ≥3 in the phase 3 PN012 trial in adolescents and global surveillance studies after administration of 200 mg of tedizolid phosphate once daily for 6 days by intravenous (a and b) and oral (c and d) routes. fAUC, area under the concentration-time curve for the free, unbound fraction of a drug; PK/PD, pharmacokinetic/pharmacodynamic.